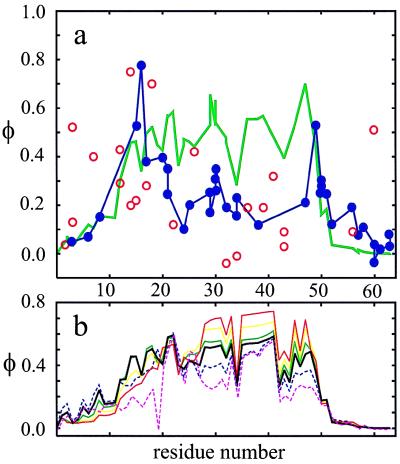
Figure 6.
Theoretical calculation of φ values. (a) Comparison of experimental and theoretical φ values for CI2. The experimental uncertainty in the φ values arises mainly from the uncertainty in the determination of the folding equilibrium constants. The φ values reported by Itzhaki et al. (40) were therefore divided into two groups according to the absolute magnitude of the change in folding free energy introduced by the mutation (at 4 M urea, the midpoint of the unfolding transition for the wild type). One group (filled blue circles) corresponds to φ values for mutations that cause folding free energy changes >1 kcal⋅mol−1 compared with wild-type whereas the second group (open red circles) corresponds to φ values for mutations that cause free energy changes <1 kcal⋅mol−1. In this second group, there are five negative φ values and one value >1.0, which are not plotted. Also, the value plotted for residue 16 is the one redetermined by Ladurner et al. (41). A blue line connects the filled blue circles to indicate that these values are, for the most part, better determined. Large changes in folding free energy can, however, change the φ value by changing the shape as well as the size of the free energy barrier. To investigate this effect, we used our model to calculate the dependence of the φ value for CI2 on the magnitude of the free energy change (b). These calculations show that the difference between the theoretical φ value calculated using the measured free energy change and the theoretical φ value calculated in the small perturbation limit (see Methods) is <0.1 for all residues except 29 and 47. (b) Dependence of theoretical φ values on the magnitude and sign of the free energy change produced by a mutation. The thick black line corresponds to φ values in the small perturbation limit. Dashed lines are φ values for mutations that stabilize the native state (magenta for |ΔΔG| = 3 kcal⋅mol−1; blue for |ΔΔG| = 1.5 kcal⋅mol−1), and continuous lines are for mutations that destabilize the native state (green for |ΔΔG| = 1.5 kcal⋅mol−1; yellow for |ΔΔG| = 3 kcal⋅mol−1; red for |ΔΔG| = 4.5 kcal⋅mol−1).