Abstract
The vertebrate ocular lens is a fascinating and unique transparent tissue that grows continuously throughout life. During the process of differentiation into fiber cells, lens epithelial cells undergo dramatic morphological changes, membrane remodeling, polarization, transcriptional activation and elimination of cellular organelles including nuclei, concomitant with migration towards the lens interior. Most of these events are presumed to be influenced in large part, by dynamic reorganization of the cellular actin cytoskeleton and by intercellular and cell: extracellular matrix interactions. In light of recent and unprecedented advancement in our understanding of the mechanistic bases underlying regulation of actin cytoskeletal dynamics and the role of the actin cytoskeleton in cell function, this review attempts to summarize current knowledge regarding the role of the cellular actin cytoskeleton, in lens fiber cell elongation and differentiation, and regulation of actin cytoskeletal organization in the lens.
Keywords: Actin cytoskeleton, Lens, Differentiation, Elongation, Migration, Signaling, Rho GTPases
Introduction
The most important function of the vertebrate lens is to focus incident light onto the retina, a function that is critically dependent on lens transparency and properties of accommodation. The ocular lens is a simple tissue encapsulated by a thick basement membrane (the capsule) and comprised of a monolayer of epithelial cells that reside only at the anterior surface, with the differentiated lens fiber cells occupying the bulk of the lens body. During development, following the formation of the lens vesicle from the surface ectoderm, cells from the anterior part of the lens vesicle differentiate into lens epithelium, while those in the posterior half elongate and differentiate into primary lens fibers. The newly divided cells at the germinative zone of the lens epithelium migrate posteriorly from the equator, elongate and differentiate into secondary fiber cells. During differentiation, epithelial cells at the equator first exit from cell cycle, elongate progressively and by several fold relative to their original size. Concomitant with the morphological changes linked to elongation, differentiating epithelial cells also initiate expression of fiber cell -specific and -abundant proteins including the crystallins, water channel and gap junction proteins, and beaded filament proteins. Finally, during terminal differentiation, lens fibers lose most cellular organelles including the nuclei through apoptosis-like mechanisms. Unlike in other tissues, while the lens does not shed any cells, newly differentiated cells are added to the lens mass on a continuous basis, in a concentric manner originating from the lens periphery (1–7).
Lens fiber cell morphology, migration, membrane remodeling and intercellular interactions are thus some of the key determinants of lens shape, symmetry and ultimately optical properties. During differentiation, epithelial cells undergo vertical elongation, with the anterior and posterior tips of the elongating fiber cells sliding along the epithelium and capsule, respectively, as these cells migrate inward. Fiber cells finally detach at the suture where they form contacts with their counterparts from the opposite side of the lens. These cellular movements are highly coordinated through actin cytoskeletal reorganization, generation of contractile force and cell adhesive interactions (1, 4, 7–15). Although important insights have emerged regarding the identity of genes controlling early stages of lens formation and lens-specific expression of crystallins (3, 5, 16), little is known about the signaling mechanism(s) regulating fiber cell migration, adhesive interactions, cell shape changes, and maintenance of fiber cell symmetry (11, 17–19). Broadly speaking, both, the identity and roles played by specific cytoskeletal structures in regulating fiber cell elongation and differentiation are presently far from clearly understood (1, 20, 21).
1. Lens actin cytoskeleton
Transparency and symmetry are unique features of the ocular lens. Lens transparency depends on several factors, most notably the organization of cytoplasmic, cytoskeletal and membrane proteins and cell-cell interactions (4, 11, 13, 20–22). Cytoskeletal proteins support an important scaffolding function for the organization of cytosolic and membrane-bound proteins in the lens, with actin-based microfilaments, microtubules and intermediate filaments being confirmed to play an essential in lens transparency (15, 23, 24).
Actin has been identified as one of the major cytoskeletal proteins in the lens and has been purified and characterized from the lens fibers of several species (14, 25, 26). The actin cytoskeleton participates in and regulates a variety of essential biological functions including morphogenesis, cell migration, polarity, adhesion, trafficking, transcription and cell survival in eukaryotic cells (27–29). In addition to providing a structural framework around which cell shape and polarity are defined, the dynamic properties of actin, and its interaction with cell adhesion proteins and plasma membrane proteins provide mechanical force driving cell movement and division (30, 31). Many of the studies conducted by Rafferty et al (32, 33) focused on the organization of actin filaments into polygonal arrays in the lens epithelium, in the context of a potential relevance in lens accommodation. The contractile activity of actomyosin and the tensile strength offered by this structure are properties believed to be important for lens accommodation and lens shape (4, 9, 34–36).
1.1 Role of lens actin cytoskeleton in fiber cell elongation and differentiation
The processes of lens fiber cell elongation and differentiation are associated with dramatic changes in cell morphology, with the length of fiber cells increasing on the order of several hundred-folds (1, 4, 7). These morphological changes are presumed to be associated with membrane cytoskeleton remodeling, actin filament assembly and cell adhesive interactions (10, 13, 14, 37–43). The ratio of F-actin to G-actin increases during lens epithelial cell elongation (39), with the actin cytoskeleton exhibiting a distinct pattern of organization (40, 44). Actin content is increased in the elongating epithelium relative to central epithelium, and increased actin stress fiber formation has been documented to occur in parallel with lens fiber cell elongation (38, 40, 44). Furthermore, disruption of the actin cytoskeleton has been shown to impair lens epithelial elongation and differentiation in several species, indicating the importance of actin filament integrity for these processes (45, 46). Actin filament organization influences lens epithelial growth by altering cell shape, with actin reorganizing to the cortical or cytoplasmic side of the plasma membrane in elongating lens fibers (47, 48). Studies from the Menko laboratory have shown that differentiation of embryonic chick lens epithelial cells is associated with changes in actin cytoskeletal organization from cytosolic stress fibers to membrane attached cortical bundles, and that this transition is associated with formation and stabilization of N-cadherin cell-cell junctions in lens fibers (12, 49–51). Importantly, recent studies from the same laboratory also revealed that the disruption of actin stress fibers in embryonic chick lens epithelial cells induces expression of differentiation markers such as δ-crystallin and filensin, and leads to an increase in N-cadherin assembly (19). Collectively these observations demonstrate a strong association between actin cytoskeletal dynamics, cell adhesive interactions and the processes driving lens fiber cell elongation and differentiation.
Actin function is determined by the ability of this cytoskeletal protein to interact with myosin II resulting in formation of actin: myosin assemblies. The actomyosin complex is a critical determinant of contractile characteristics of both nonmuscle and smooth muscle cells, and regulates cell motility, cytokinesis, formation and stability of cell adhesions and junctions (52–54). Myosin II forms bipolar filaments, enabling it to contract actin-filament networks in the cytoplasm, thereby influencing membrane tension. Myosin II is present in lens epithelial and cortical fiber cells and also in the lens fiber cell basement membrane complexes (9, 14, 40, 55). Our recent work on the distribution pattern of myosin II in lens revealed distinct spatial profiles for myosin IIA and IIB. While myosin IIA was distributed uniformly throughout the developing lens, including in the epithelium and differentiated fibers, myosin IIB was localized predominantly to the epithelium and the posterior tips of the lens fibers. Furthermore, elongating lens fibers revealed marked enrichment for phosphorylated myosin II (which is the activated form of myosin II) relative to undifferentiated lens epithelium, indicating a potential association between actomyosin reorganization and fiber cell differentiation (Maddala, Skiba and Rao, submitted). Interestingly, a mutation in nonmuscle myosin IIA is linked to development of cataract in humans (56). Our recent data from microarray analysis of a cDNA library from a 7-day old mouse lens revealed that myosin light chain is one of the abundantly expressed genes, indicative of a potentially significant role for this protein in the lens.
Tropomodulin, a tropomyosin and actin-binding protein that stabilizes tropomyosin-actin filaments by capping pointed ends of actin, is an important component of the lens microfilament cytoskeleton. Tropomodulin is expressed selectively in the differentiated lens fibers and assembles onto the plasma membrane only after fiber cells have begun to elongate, and form inter-apical contacts with the undifferentiated epithelium (13, 57–59). This suggests that actin filament capping by tropomodulin is critical for remodeling of the lens membrane skeleton during fiber cell differentiation. Based on their unique interactions with F-actin and other cytoskeletal proteins in the differentiating lens epithelial cells, tropomodulin and tropomyosin are believed to be involved in remodeling and stabilizing actin cytoskeletal organization during lens fiber cell differentiation (13, 58).
1.2. Lens actin cytoskeleton and fiber cell migration
Directed cell migration and polarity depend on asymmetric cues that lead to reorganization of the cytoskeleton and polarized localization of several cortical proteins that serve as downstream effectors in transducing external signals into intracellular responses. On the mechanistic level, directed cell migration depends on lamellipodium extension, formation of focal adhesions, and generation of contractile force, followed by a process of retraction (29, 31, 60–63). As lens epithelial cells at the equatorial zone start elongating, they also migrate posteriorly. The elongated fibers move posteriorly along the capsule, and anteriorly along the fiber cell-epithelial interface, to generate a symmetrically organized fiber cell mass. The controlled and directed migration of lens fibers is crucial to the formation of ordered suture patterns, thereby optimizing lens optical quality (1, 4, 7, 9, 11, 13). The dynamic assembly of actin filaments that underlies generation of force and drives interactions of cells with the extracellular matrix, is critical for the control of cell migration (29, 62). At the points of contact between cells and the extracellular matrix, transmembrane integrin adhesion receptors assemble to constitute focal adhesions which provide a structural link between the actin cytoskeleton and the extracellular matrix (9–11, 13, 49, 64–71).
Bassnett et al. (9) have reported that the lens fiber cell basal membrane complex (BMC) which anchors fiber cells to the lens capsule, and facilitates their migration across the capsule, is enriched in contractile and cell adhesive proteins including myosin, myosin light chain kinase, actin filaments, caldesmon, paxillin and focal adhesion kinase (FAK). Blanquet and Courtois (71) have reported distinct changes in BMC cytoskeletal assemblies between differentiating and undifferentiating lens epithelial cells. Al-Ghoul et al (72) also provided further support for the involvement of actin filaments in BMC organization at lens fiber ends and suggested that the shape and arrangement of fiber ends varies in a predictable pattern during migration. This work also indicated that elongating fiber ends follow defined migration patterns along the posterior capsule to their sutural destinations. Beebe et al (10) have shown that the organization and composition of adhesion complexes of lens fiber cells change during lens fiber cell elongation. Staining for vinculin and paxillin for instance, shows increases specifically in differentiated chick embryo lens fibers, but is reduced in elongating fibers, indicative of regulated expression and differential function of focal adhesions during distinct stages of fiber cell differentiation. Moreover, increased adhesive complexes are believed to stabilize cell-cell interactions during fiber cell maturation. Studies from the Fowler laboratory reported that tropomodulin, a lens fiber-specific actin-capping protein, assembles uniquely at the apical and basal ends of newly differentiated fiber cells, and that this localization disappears once fiber cells form sutures, supporting a potential role for membrane-bound actin cytoskeleton in polarity and migration (13).
A direct line of evidence for the significance of lens fiber cell migration in lens development and differentiation comes from a recent study by Grove et al, (73) in which homozygous deletion of Abi-2, a c-abl kinase interacting protein and regulator of actin dynamics, has been shown to lead to defective lens fiber cell migration and orientation. The c-abl-interactor (Abi) family of adaptor proteins has been linked to signaling pathways involving the Abl tyrosine kinases and the actin cytoskeleton. Both Abi-1 and Abi-2 localize to the adherens junctions and to the leading edges or lamellipodia of cells (73, 74). Abi-1 and Abi-2 together with c-abl kinase regulate actin dynamics in response to stimulatory input from an activated Rac GTPase. c-abl and Abi-1/Abi-2 bind to and phosphorylate WAVE (N-WASP related protein), thereby modulating WAVE activity and inducing actin polymerization via Rac GTPase activation (75–78). Abi-2 has been shown to localize at the tips of the elongating secondary lens fibers and at the adherens junction, colocalizing with cadherins and β-catenin (73). In primary lens epithelial cells, we showed that both Abi-1 and Abi-2 localize to the lamellipodia and to the adherens junctions. Further, WAVE-1, Arp2/3, abi-2 and α-crystallin all colocalize to the leading edges of migrating lens epithelial cells, indicating their involvement in cell migration (68).
1.3 Lens cell adhesion proteins and actin cytoskeleton
The integrin family of extracellular matrix receptors regulates many aspects of cell function, in particular cell adhesion and migration. These two processes depend on organization of the actin cytoskeleton into adhesive and protrusive organelles in response to extracellular signals. Integrins are important switch points for the spatiotemporal control of actin-based motility in higher eukaryotes. Interaction of integrin receptors with extracellular ligands drives the assembly of complex multiprotein structures that link the ECM to the cytoplasmic actin cytoskeleton. These adhesive complexes are dynamic, often heterogeneous structures, varying in size and organization (29, 31, 79, 80). Signal transduction through these adhesion complexes has been implicated in the regulation of a number of key cellular processes; including growth factor induced mitogenic signals, cell survival and cell locomotion (79). Lens epithelial and fiber cells from different species have been shown to express fibronectin and laminin binding receptors including Alpha3A, alpha6A, alpha6B, beta 1A, beta3 and alpha5beta1 (9, 11, 49, 81–85). Importantly, some of these integrins are known to exhibit distinct shifts in expression pattern during fiber cell differentiation. Notably, while expression level of alpha6B integrin is down regulated, that of alpha6A integrin is upregulated during fiber cell differentiation, with this shift being associated with a change in actin cytoskeletal organization. Alpha6A integrin exhibits increased binding to the actin cytoskeleton in differentiating lens fibers (49, 51, 83). Interestingly, region-specific differences have been documented in expression profiles of cytoskeletal proteins involved in cell-substrate interactions and signaling proteins, between epithelial cells which have just embarked on the path to differentiation versus those that are located at the central epithelium (44, 49, 70, 71).
Cadherins are intercellular adhesion receptors that are essential for the establishment of the epithelial cell shape and maintenance of the differentiated epithelial phenotype. In order to support efficient intercellular adhesion, cadherin receptors require association with actin filaments (86–88). N-cadherin is the most abundant adherens junction protein in the lens (12, 41, 69, 89). Studies by Geiger et al (67, 90) have demonstrated the importance of actin cytoskeleton interactions with adherens junction proteins in lens epithelial cells. Lens epithelial cells have been shown to form A-CAM-dependent adherens junctions, which in turn reveal a dependence on actin microfilament organization (91, 92). Lens epithelial cells and fiber cells express cadherin and β-catenin which are engaged in formation of cell-cell adhesions. Interestingly, inhibition of Rho GTPase activity both in lens epithelial cells and in lens fibers disrupt β-catenin-based adherens junctions, concomitant with depolymerization of the actin filament network, indicating the importance of the actin cytoskeletal organization in regulating adherens junction formation (18, 93). Based on immunofluorescence electron microscopy, Lo has reported the distribution of adherens junctions existing in complexes with actin filaments, and implicated a potentially important role for such interactions in the accommodative properties of the lens (94). During lens differentiation both N- and B-cadherins exhibit a progressive association with cytoskeletal proteins (89). Recently, Strub et al. (69) have demonstrated the existence of two types of abundant cortical adherens complexes in lens fibers: Type-1 complexes containing largely N-cadherins, cadherin-11, α and β-catenin, plakoglobin, p120 catenin and vinculin, and Type II complexes containing ezrin, periplakin, periaxin and desmoyokin (EPPD), usually together with moesin, spectrin and plectin. These two types of membrane cortical complexes are organized in an alternating manner along the fiber cell membrane suggesting a critical role for these complexes in mediating fiber cell organization and interactions. NrCAM cell adhesion proteins, which bind to ankyrin-B, are found to be essential for lens integrity, and a homozygous deletion of NrCAM in the mouse leads to development of cataract and abnormalities in organization of the actin cytoskeleton (95).
Finally, the decision of lens epithelial cells to withdraw from the cell cycle and initiate differentiation has been shown to depend on the inhibition of src family of kinases and the formation of N-cadherin cell-cell junctions (96). These changes are also associated with reorganization of actin cytoskeleton to the cortical regions of lens cells (12). Chick lens epithelial cells treated with anti-N-cadherin antibody fail to express differentiation-specific markers (50). These observations suggest that N-cadherin and the actin cytoskeletal structures associated with the N-cadherin complexes play an important role in the differentiation process that leads to the formation of the crystalline lens (12).
1.4 Lens actin interacting proteins
The ezrin/radixin/moesin (ERM) family of actin-binding proteins which serve as both, linkers between the actin cytoskeleton and plasma membrane proteins, and as signal transducers of actin cytoskeletal remodeling events, are involved in cell-cell adhesion, maintenance of cell shape, cell motility and membrane trafficking (97, 98). Ezrin and radixin have been shown to be present in elongating cortical lens fibers (43). ERM proteins are especially important for cortical actin organization and growth signaling (99). The closely related tumor suppressor, merlin, shares many properties with ERM proteins (99, 100). Merlin and the ERMs have been shown to interact with and regulate N-WASP, a critical regulator of actin dynamics (100). Straub et al (69) have demonstrated the existence of abundant cortical adherens complexes in lens fibers containing the ezrin complexes. The ERM proteins and merlin regulate Rho GTPase activity by regulating the release of Rho GDI from Rho GTPases, which in turn modulates actin cytoskeletal organization (101). Although the molecular mechanism linking the mutation in the merlin gene product with cataract development is not clear, the observed similarity of function with the ERM proteins strongly supports a role for merlin function in lens function and transparency (102).
α-crystallin, a small heat shock protein which displays the properties of a molecular chaperone, and is present in both lens epithelial and fiber cells, is another well recognized actin binding protein (103). A substantial proportion of α-crystallin exists in association with the plasma membrane cytoskeleton in lens fiber cells (14, 104, 105). α-crystallin has been shown to localize to leading edges of migrating lens epithelial cells where actin polymerization is dynamically regulated by various actin-binding proteins. Further, in lens epithelial cells, α-crystallin colocalizes perfectly with WAVE, an important activator of Arp2/3 complex nucleation activity (68). In lens fiber cells, actin also colocalizes with the gap junctions, aquaporins and membrane interdigitations indicating a potential role for actin in membrane interactions and organization (106–110). Actin also belongs to a group of proteins that is targeted for calpain-mediated degradation in different types of experimental cataract (20, 111). Taken together, these observations indicate a critical importance for actin in providing a scaffolding function, and in organization of soluble crystallins and membrane-associated proteins, which are in turn key determinants of lens transparency.
2. Regulation of lens actin cytoskeletal organization
Although it is well recognized that several external stimuli such as growth factors and hormones influence lens epithelial cell proliferation and fiber cell elongation and differentiation, very little is known regarding the mechanistic bases by which these processes are regulated (1, 3, 6). The actin cytoskeleton and actin-interacting proteins conceivably play a vital role in many of the cellular changes associated with lens fiber cell elongation and differentiation through the ability of actin to reversibly polymerize, generate contractile forces, and interact with structural and functional (signaling) membrane proteins (11–13, 112). Research conducted in our laboratory over the last couple of years has focused on understanding the regulation of actin cytoskeletal signaling and its role in lens fiber cell elongation and differentiation (17, 18, 68, 112, 113). Members of the Rho GTPase family of small GTP-binding proteins are recognized to serve as master control switches of actin cytoskeletal organization and regulation of various actin-dependent cellular processes in eukaryotic cells (28, 30, 114, 115). Using both pharmacological and molecular approaches, we evaluated the regulation of Rho GTPase activity in lens epithelial cells, and explored the potential role of the Rho-GTPases in lens development and function. In addition to Rho GTPases, calcium dependent pathways, (116), src kinases (96), PI3 kinase (117), Cdk5 (118), PDZ domain proteins (119), c-abl interactor proteins (73) and Wnt/frizzled signaling (120, 121) pathways, all of which influence actin cytoskeletal dynamics and cell adhesions, are also considered to be important for lens cell migration, elongation and differentiation.
2.1 Brief overview of Rho GTPase signaling pathways in the lens
Rho GTPases are GTP-binding proteins that control many features of cell behavior in all eukaryotic cells. They play a pivotal role in regulating actin cytoskeletal organization, and influence cell polarity, contraction, migration, membrane transport pathways, cell cycle progression and transcriptional activity (28, 30). The Rho GTPases (Rho A, B and C, Rac 1, 2, and 3, and Cdc42) comprise a large subfamily of the Ras-superfamily of GTPases and are highly conserved in eukaryotic oganisms. The most well-understood and studied functions attributed to the Rho GTPases include formation of actin stress fibers and focal adhesions, cell motility (Rho GTPase), regulation of membrane ruffling, lamellipodium formation, actin polymerization, and cadherin-mediated cell-cell adhesion (Rac GTPase), filopodium formation, and microtubule-dependent cell polarization and migration (Cdc42). Based on these activities it is widely accepted that the Rho GTPases play a critical role in cellular processes that are dependent on actin cytoskeletal organization (28, 30, 114, 115, 122). The Rho family of GTPases acts as molecular switches by cycling between an active GTP-bound and an inactive GDP-bound form. This cycling is regulated by the guanine nucleotide exchange factors (GEFs) which serve as Rho GTPase activators, while the GTPase activating proteins (GAPs) and GDP dissociation inhibitors (GDIs) act as negative regulators. In the GTP-bound form, the Rho GTPases interact with specific downstream effector proteins, which include protein kinases, regulators of actin polymerization, and adapter proteins. The selective interaction of the different Rho GTPases with a variety of downstream signaling effectors determines the final outcome of Rho GTPase activation (30, 115). The interaction of the Rho isoforms RhoA and RhoB with the ROCK family of kinases or mDia1 affects actomyosin organization and contractile force, or stimulates actin polymerization, respectively (123). The p21-activated kinase (PAK) family of proteins acts down stream of both Rac and cdc42, affecting actin organization (124). Additionally, Rac GTPase regulates activity of the Arp2/3 complex via WAVE/Sra-1/Abi-1, and induces actin polymerization (76), while Cdc42 regulates actin dynamics through the Wiskott-Aldrich syndrome protein (WASP) (78). Both Rho kinase and PAK kinase activate LIM kinase, which in turn inactivates the actin depolymerizing activity of cofilin through phosphorylation of the latter protein (114, 125). Rho GTPases control other cellular activities, such as that of the JNK (c-jun N-terminal kinase) and p38 MAPK (mitogen-activated protein kinase) and G1 cell cycle progression (30). Rho GTPases also participate in transcriptional activation by regulating the activities of serum response factor (SRF) and nuclear factor-κB (NF-κB) transcription factors, leading to changes in gene expression (30, 126). Expression of these transcription factors is influenced by changes in actin cytoskeletal organization (126). The Rho GTPases are also involved in control of microtubule dynamics through their down stream effectors mDia3, IQGAP1 and Par 6 (30, 123, 127).
The activity of the Rho GTPases is regulated by signals originating from different classes of cell surface receptors, including the seven transmembrane domain, heterotrimeric G protein-coupled receptors, tyrosine kinase receptors, cytokine receptors, frizzled receptors, and adhesion receptors (52, 128). Various growth factors and other external factors influence the activity of Rac and Rho GTPases via modulating the respective nucleotide exchange factors which catalyze the displacement of GTPase bound-GDP by GTP (28, 114). Furthermore, growth factors that activate PI3-kinase and Src activities also regulate Rho GTPase activity, while the PKC activator PMA has been shown to activate Rac GTPase (28). Interestingly, the Wnt family of secreted signaling molecules, which are involved in maintaining cell polarity and supporting migration, mediate their effects through Rho and Rac activation (129, 130).
A subset of G protein-coupled receptors (GPCRs), including the endothelin-1, thrombin, angiotensin II, and lysophospholipid receptors, induces the formation of actin stress fibers, focal adhesions, and cell-rounding through Rho GTPase-dependent pathways (52, 128). Additionally, the heterotrimeric G-proteins, Gα12/13 and Gαq are also recognized to modulate actin cytoskeletal organization, cell-ECM interactions, and myosin II phosphorylation via activation of Rho GTPases (52, 128), in a GPCR-dependent manner. Gα12/13, which is considered to be a major upstream regulator of RhoA activity (128), mediates GPCR-driven activation of Rho A via stimulating the activity of the Rho GTPase guanine nucleotide exchange factors (p115-RhoGEF, PDZ-RhoGEF, and leukemia-associated RhoGEF, or LARG), which promote the exchange of Rho-bound GDP with GTP (52). In addition to GPCRs and receptor tyrosine kinases, several classes of cell adhesion molecules, including the integrins and cadherins, modulate Rho GTPase activity in a growth factor-responsive manner (28) (Figure 1).
Figure 1.
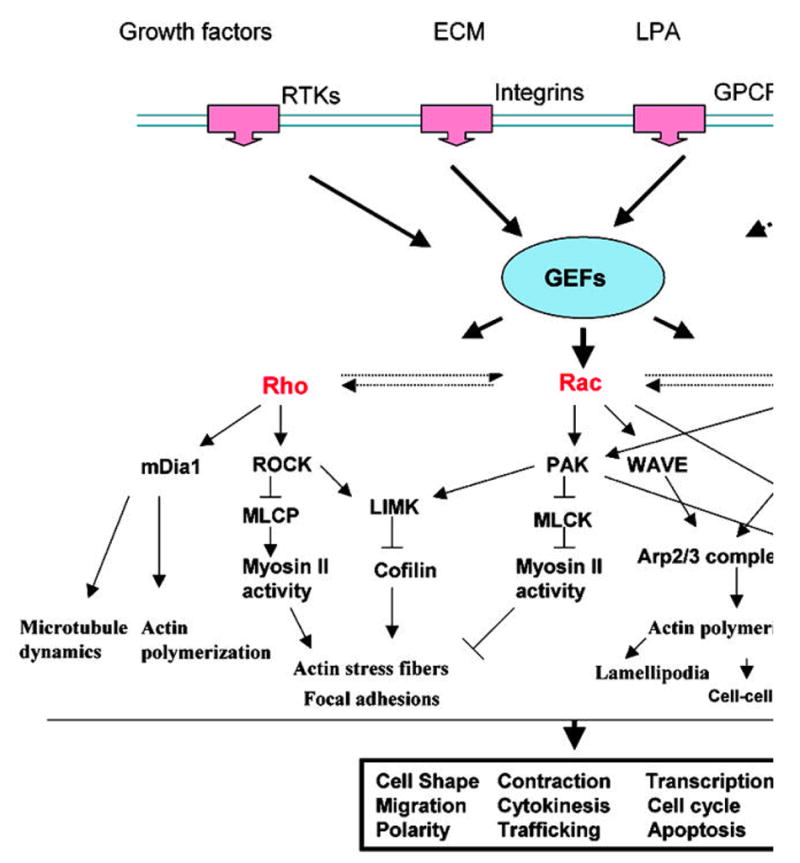
Rho GTPase-mediated signaling events involved in the regulation of actin and microtubule cytoskeletal organization. Transmembrane receptors and their ligands (top) activate Rho GTPases through GEFs (guanine nucleotide exchange factors). Activated Rho GTPases in turn activate ROCK (Rho kinase) and PAK (p21 activated kinase) kinases, which then bind to various adaptor proteins (WAVE, N-WASP, mDia, Par6 and IQGAP1) that regulate actin polymerization and microtubule assembly. The Rho activated ROCK and Rac/Cdc42 GTPases activated PAK, in turn influence MLCP (myosin light chain phosphatase) and MLCK (myosin light chain kinase activity) activities, respectively, and regulate myosin II activity via the phosphorylation of myosin light chain. Cross-talk between the Rho, Rac and Cdc42 pathways is shown by dashed arrows. Various cellular processes that are regulated by Rho GTPase activity are indicated in the box. The involvement of specific GEFs in Wnt/Frizzled-mediated stimulation of Rho GTPase activity has not been definitively established, as indicated by “?” symbols in the scheme.
2.2. Rho GTPases in lens
Since most eukaryotic cells use a combination of actin-myosin-based contraction and actin polymerization- based protrusion to regulate shape and motility, it is reasonable to predict that regulation of actin cytoskeletal organization and dynamics would be a critical determinant of lens growth and development. Based on this premise, we hypothesized that the Rho family of GTPases, and Rho, Rac and Cdc42 in particular, play a pivotal role in the processes of lens fiber cell elongation, migration and differentiation during lens development and growth. By way of preliminary work, we characterized the expression profiles of Rho (RhoA and RhoB) and Rac1 GTPases in primate and rodent lenses, and lens epithelial cells, using immunblotting, GTP-overlay and RT-PCR techniques (64, 65). As an extension of the same studies, our laboratory also evaluated the expression of proteins which regulate Rho GTPase function, including the guanine nucleotide exchange factors (Vav-1, Vav-2, Lfc, Lsc and GEF1), GTPase activating proteins (RhoGAP190, GAP129), RhoGDI (GDP dissociation inhibitor), Rho kinases (ROCK 1 and ROCK 2) and p21-activated kinase, in human and mouse lenses and in a human lens epithelial cell line (SRA-01/04). During the course of these studies, we also documented the presence of some unique but yet to be characterized small GTP-binding proteins (apparent molecular weight > 21 kDa) which exhibited an apparently selective association with the fibers of primate lenses (64). Importantly, RhoB revealed developmentally regulated changes in expression levels in the rodent lens (65). Recently, Chen et al (131) reported that while the expression of RhoA is restricted to the epithelium and differentiating lens fibers in the mouse lens, Rac and CDC42 expression is uniformly distributed throughout the lens, including the epithelium and fibers.
2.3 Rho GTPases in lens development and function
To explore the significance of Rho GTPase activity in lens function and in lens development and growth, we utilized both pharmacological and genetic approaches. An interesting feature of Rho GTPases functionality is that the activity of these proteins is dependent on membrane localization. Most members of the Rho GTPase family are posttranslationally isoprenylated at the C-terminus, a modification which anchors them to membranes (28, 132). The endogenous biosynthesis of isoprenoids, which are intermediates in the cholesterol biosynthetic pathway, can be inhibited by the statins, a group of 3-hydroxy-3-methylglutary coenzyme A (HMG-COA) reductase inhibitors that have been widely used in the treatment of hypercholesterolemia (133). Isoprenylation of Rho GTPases is mediated by geranylgeranyl transferase. The statins and specific inhibitors of geranylgeranyl transferase have therefore been exploited as pharmacological tools to explore Rho GTPase function in various cell types (132). In experiments using organ cultured rat lenses, we noted that lovastatin treatment results in development of subcapsular and cortical opacity in association with extensive morphological changes in cortical fibers. In human and porcine lens epithelial cells, lovastatin induces dramatic changes in cell shape, including a profound loss of actin stress fibers, focal adhesions, protein phosphotyrosine, and beta-catenin associated adherens junctions. These effects of lovastatin are associated with accumulation of nonisoprenylated Rho and Rac GTPases in the cellular cytosolic fraction. Furthermore, supplementation of culture media with geranylgeranyl pyrophosphate dramatically reversed the lovastatin-induced morphological and cytoskeletal changes indicating that impairment of geranylgeranylated Rho and Rac GTPase function is most likely responsible for the lovastatin-induced cytoskeletal changes in lens epithelial cells (93, 113, 134). Interestingly, Girao el al (135) have recently reported that cholesterol oxide induces cytoskeletal changes including actin-based microfilament reorganization in a Rho, Rac and Cdc42 –dependent manner in lens epithelial cells, further supporting the importance of Rho GTPases in regulation of lens cytoskeletal dynamics.
Statins are commonly used cholesterol lowering drugs generally lacking in harmful side effects. Higher concentrations of statins, however, consistently cause cataracts in various experimental models and long-term use of statins is suggested to increase risk for cataract development in humans as well (113). The clinical condition of Mevalonic aciduria, which is a consequence of the deficiency of mevalonate kinase (the first enzyme following the 3-hydroxy-3-methylglutaryl-coenzyme A reductase-catalyzed step in cholesterol and nonsterol isoprene biosynthesis) is commonly associated with cataract formation in humans (136).
To obtain more direct evidence for the involvement of Rho GTPase in lens growth and function in vivo, we expressed the Rho GTPase specific inhibitor C3-exoenzyme, in a lens-specific manner in transgenic mice by using the lens-specific αA-crystallin promoter. C3-exoenzyme, a bacterial toxin from Clostridium botulinum, is a specific inhibitor of RhoA, B and C isoforms and is used extensively as a pharmacological tool to modulate Rho GTPase activity. In the presence of NAD, C3-exoenzyme ADP-ribosylates and irreversibly inactivates Rho GTPases (28, 114). Expression of the C3 exoenzyme transgene in the mouse lens was associated with a specific ocular phenotype including cataract, microphthalmia and ocular hemorrhage. While lenses obtained from these mice exhibited normal expression of RhoA and RhoB, the level of functionally active Rho GTPase was significantly reduced, as confirmed by in vitro ADP-ribosylation assays using 32P labeled NAD (112). Lenses from these mice also revealed extensive damage to the lens including a ruptured posterior capsule, thickened anterior lens capsule, abnormal fiber cell shape and migration, extensive vacuolization in anterior cortical fibers and accumulation of nuclei in differentiated fibers suggesting defects in terminal differentiation (Figure 2). Lens weight in C3 mice was reduced to 50% of that observed in control mice and increased apoptosis was noted in both cortical and nuclear regions of day1 C3 lens. Immunostaining for actin stress fibers, adherens junctions, the gap junctional protein connexin-50, and water channel protein MIP-26 was noticeably reduced in lens fibers of C3 transgenic mice as compared to littermate controls, indicative of alterations in cytosketal organization and fiber cell-cell interactions (18). The histological, cytoskeletal and biochemical data obtained from this transgenic model provided strong evidence for a critical role for Rho GTPase activity in lens development, growth and function.
Figure 2.
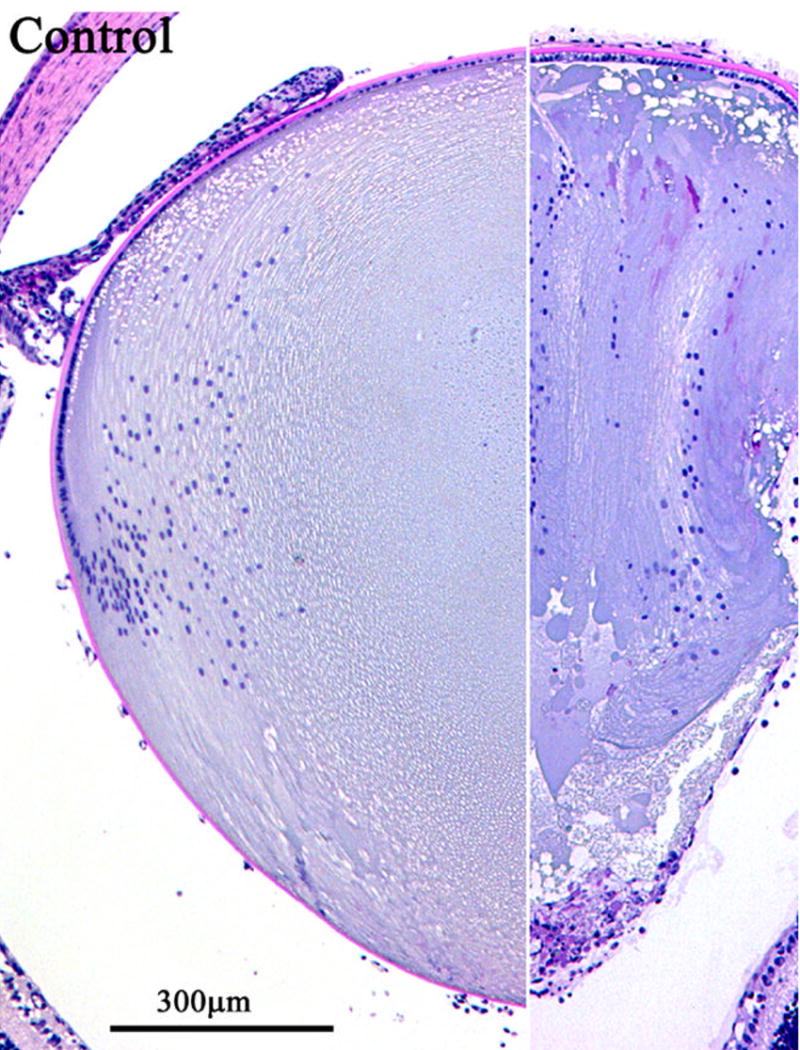
Inactivation of Rho GTPase function disrupts lens growth, structure and function in a transgenic mouse model expressing the C3-exoenzyme. A transgenic mouse expressing the C3-exoenzyme in a lens specific manner, develops cataract and exhibits abnormal fiber cell migration, organization and differentiation (18). C3-exoenzyme, is a bacterial protein that inactivates Rho GTPase activity through ADP-ribosylation. The C3 gene was placed under the control of an alpha-A crystallin promoter to drive lens –specific expression of this Rho GTPase inhibitor. Histological sections of lenses from 7 day old littermate control and transgenic (TG) mice were stained with Periodic acid Schiff reagent (PAS). The resulting micrographs are aligned in such a way that the same spatial regions can be compared between control and transgenic specimens.
Recent studies in our laboratory have generated additional and independent support for the involvement of Rho GTPases in lens development and growth via analyzing the effects of overexpression of Rho GDI, a negative regulator of both Rho and Rac GTPase function, in lenses of transgenic mice (Rao and Maddala unpublished).
2.4 Rho GTPases in growth factor signaling in lens epithelial cells
Among the various external cues that influence lens fiber cell elongation and differentiation, growth factor receptor-initiated signaling has been well recognized as a critical input. Various classes of tyrosine kinase receptors and their cognate ligands (PDGF, EGF, FGF, TGF-β, and IGF) play key roles in the initiation, progress and maintenance of the lens epithelial cell proliferation and differentiation programs (6, 137). Since these processes are closely tied with cell cycle progression, migration, and changes in cell morphology, we explored the involvement of the Rho GTPase-mediated signaling pathway in growth factor-stimulated actomyosin cytoskeletal reorganization and focal adhesion formation in lens epithelial cells. To do this, we evaluated the effects of different growth factors including EGF, b-FGF, PDGF, TGF-β, and IGF-1 on activation of Rho and Rac GTPases in human lens epithelial cells. Both Rho and Rac GTPases are activated in human lens epithelial cells by EGF, b-FGF, TGF-beta and IGF-1, as determined by pull-down assay-based analysis of the GTP-bound form of GTPases (17). Under these conditions, the activation of Rho GTPase is associated with a strong increase in cortical actin stress fibers and integrin-mediated focal adhesions (Figure 3). Further, the growth factor-induced changes in actin cytoskeletal and cell adhesive properties are inhibited by C3-exoenzyme, lovastatin, or the Rho kinase inhibitor Y-27632, supporting the involvement of Rho GTPases in these events (17). Thus, we speculate that the ability of growth factors to trigger activation of Rho and Rac GTPases, together with actomyosin cytoskeletal reorganization, formation of focal adhesions and adherens junctions, plays a crucial regulatory role in lens epithelial cell proliferation, migration, elongation and differentiation.
Figure 3.
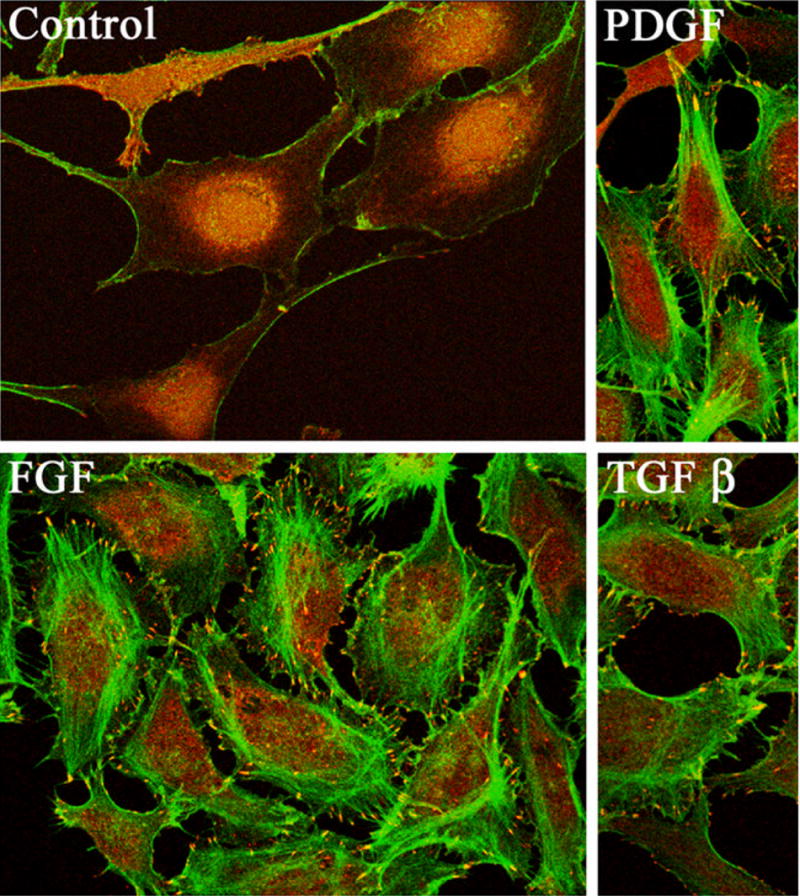
Growth factor-induced reorganization of the actin cytoskeleton and formation of focal adhesions in human lens epithelial cells. This figure depicts the FGF, PDGF and TGF-β-induced actin cytoskeletal reorganization (formation of actin stress fibers and cortical actin bundling) and focal adhesion formation in serum starved human lens epithelial cells (17). Cells were double-labeled with rhodamine-phalloidin for actin and with vinculin antibody in conjunction with fluorescein isothiocyanate-conjugated secondary antibody for focal adhesions.
The lens expresses several GPCRs whose activity has been implicated in the regulation of calcium fluxes or the calcium current in this ocular tissue (116). Bioactive lysophospholipids (sphingosin-1-phosphate and lysophosphatidic acid) play key roles in the regulation of fundamental cellular processes including proliferation, survival and migration. LPA is one of the external factors recognized early on to influence actin cytoskeletal organization and cell adhesions in various cell types. Both LPA and SIP activate intracellular signaling proteins including PI3 kinase, AKT, MAP Kinases and the Rho and Rac GTPases, via engaging the endothelial differentiation gene (Edg) family of G-protein coupled receptors (138). Lysophospholipids are present in various biological fluids including the serum and aqueous humor (139). The role of these lipid growth factors and their receptors in lens epithelial cell proliferation and differentiation is far from understood. Interestingly, in Rho GTPase functional knockout transgenic mouse lenses, we detected a four- fold increase in the expression of the EDG -2 receptor (18), and also confirmed expression of various EDG receptors in human lens epithelial cells and in human and murine lens tissue by RT-PCR analysis. Furthermore, we noted that in serum starved human lens epithelial cells, both LPA and SIP induce formation of actin stress fibers, focal adhesions, and stimulate increases in AKT phosphorylation in association with activation of Rho and Rac GTPases (17, 140). These observations provide important insights into a potential role for lysophospholipids in lens epithelial cell migration, elongation and differentiation. Thrombin is another well recognized agonist which activates Rho GTPase and calcium-dependent signaling pathways in target cells (28, 52). Lens epithelial cells express thrombin receptors which regulate calcium signaling in these cells (141). Thrombin also induces formation of actin stress fibers, cell adhesions and cellular contraction in lens epithelial cells. Additionally, endothelin-1, neuropeptide bombesin, and agonists of alpha-adrenergic receptors, all of which regulate Rho GTPase activity and actin cytoskeletal dynamics through GPCRs (52), also modulate the calcium current in lens epithelial cells suggesting that these agonists might play a role in driving lens epithelial cell migration, contractile activity and differentiation through regulation of cytoskeletal reorganization and cell adhesive interactions (141).
Activated myosin II interacts with actin to form actomyosin based stress fibers which generate mechanical force through contraction. Myosin light chain is a regulatory subunit of myosin II, and the phosphorylation status of this protein determines the activity of myosin II ATPase (53). Myosin II phosphorylation is regulated by both calcium-dependent myosin light chain kinase and calcium-independent Rho kinase (52). EGF, TGF-β, LPA and TNF-α stimulate MLC phosphorylation in serum starved lens epithelial cells in Rho kinase-dependent manner (Figure 4). Additionally, overexpression of DNRK in lens epithelial cells completely suppresses basal levels of MLC phosphorylation, an effect that is associated with decreased actin stress fibers and focal adhesions (142). Further, several growth factors are known to modulate the calcium current in lens epithelial cells (11). These observations also indicate the importance of growth factor signaling in actomyosin reorganization and assembly of cell adhesions mediated through the activation of the Rho/Rho kinase pathway and calcium mobilization in lens epithelial cells. Additionally, TGF-beta has been shown to induce expression of alpha smooth muscle actin, and stimulate cellular contraction in lens epithelial cells. Overexpression of a dominant negative TGF-beta receptor leads to an impairment of actin filament assembly in lens epithelial cells (143, 144).
Figure 4.
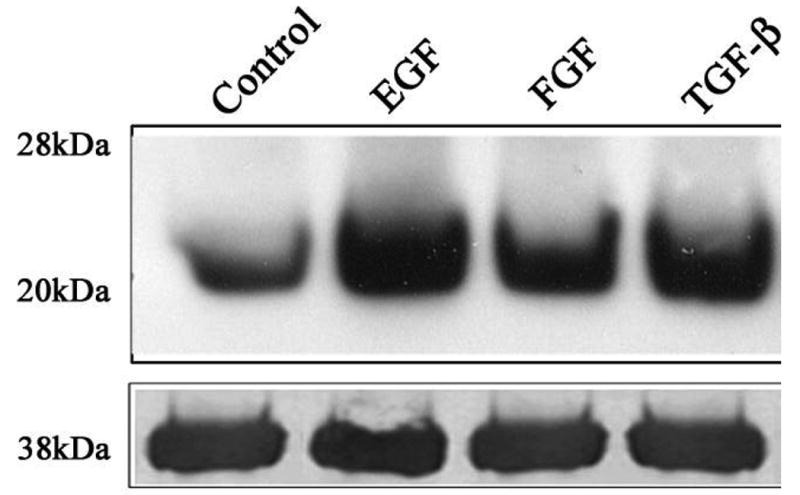
Growth factor-induced myosin light chain phosphorylation in primary lens epithelial cells. To determine the effects of growth factors on regulation of MLC phosphorylation in lens epithelial cells, serum starved porcine lens epithelial cells were stimulated with EGF (20 ng/ml), b-FGF (20 ng/ml), TGF-β (20 ng/ml), LPA (5 μg/ml) or TNF-α (1 ng/ml) for one hour. Cell lysates were analyzed for changes in MLC phosphorylation by Western blot analysis using anti-phosphospecific MLC polyclonal antibodies. The same samples were also probed for glyceraldehyde 3-phosphate dehygrogenase (G3PDH), to confirm loading equivalency.
Several growth factors activate PI3 kinase in lens epithelial cells, and this enzyme is recognized to be differentially regulated in epithelial cells versus differentiating lens fiber cells (117, 145). Growth factor activation of PI3 kinase in turn drives activation of the guanine nucleotide exchange factors (Vav, Tiam-1 and PIX) which subsequently stimulate Rac GTPase activity (146). Although the effects of PI3 kinase activation on lens actin cytoskeletal organization or cell migration have not been tested directly, PI3 kinase and its product phosphatidylinositol 3,4,5-trisphosphate play a fundamental role in actin cytoskeletal dynamics, and are widely implicated in controlling cell migration and polarity (60, 146). Inositol triphosphate (IP3) has been shown to stimulate increases of intracellular calcium in lens epithelial cells and induce changes in actin cytoskeletal organization (147). IP3 induced actin cytoskeletal changes are linked to activation of PKC and calcium signaling, and both of these responses in turn activate myosin II phosphorylation, leading to contraction and an increase in formation of actin stress fibers (52). H-7, a broad serine-threonine kinase inhibitor which affects actin cytoskeletal organization, has been shown to affect cell-cell junctions in lens epithelial cells in a calcium-independent manner (90). Src kinases have been shown to influence lens differentiation in association with actin cytoskeletal reorganization (12). Src kinase and focal adhesion kinase act upstream of Rac GTPase to regulate formation of cell adhesions (28). Activation of PKCα by EGF has been found to increase phosphorylation of tropomodulin, which enhances the interaction of tropomodulin with membrane-bound cytoskeletal proteins suggesting that growth factor-mediated activation of PKC might contribute to driving cytoskeletal reorganization in lens fibers (148).
Abl-kinase and its interacting proteins, the Abi-1and Abi-2, have been recognized to participate in growth factor-mediated activation of the WAVE-Arp2/3 complex in a Rac GTPase- dependent manner (149). Homozygous deletion of murine Abi-2 causes cataract development with obvious defects in fiber cell migration and orientation (73). Additionally, Abi-2 null mice with heterozygous abi-1 deletion exhibit much more severe lenticular changes than Abi-2 alone, indicating the functional significance of both proteins in lens development and growth (Maddala, Pendergast and Rao unpublished). Importantly, Abi-1 expression has been shown to be regulated by growth factors, especially EGF, and over expression of Abi-1 inhibits ERK kinase signaling and cell proliferation (150). These observations support a role for c-abl and its binding proteins Abi-1 and Abi-2 in the regulation of cytoskeletal dynamics at adherens junctions in the lens, a mechanism which may be of critical importance for fiber cell-cell interactions and migration.
2.5 Other Signaling pathways involved in regulation of lens actin cytoskeletal reorganization and cell adhesive interactions
Cdk 5, a cyclin-dependent kinase, is expressed in neuronal cells and at low levels in other cell types including the lens (118, 151). Cdk5 localizes to the tips of elongating lens fibers, and influences cell adhesive activity in lens epithelial cells (118). Interestingly, p39, a regulator of Cdk5, has been shown to interact with muskelin, a cytoskeletal interacting protein (152). Cdk 5 has also been demonstrated to colocalize with Rac and Pak1 at the leading edges of migrating cells and to phosphorylate and inactivate PAK1 (153). These observations indicate a potential role for Cdk5 in cell migration and formation of cell adhesions in lens epithelial cells.
Lowe syndrome is a rare X-linked disorder characterized by bilateral congenital cataracts (154). This syndrome results from mutations in the OCRL1 gene, which encodes a phosphatidylinositol 4, 5 bisphosphate 5-phosphatase located in the trans-Golgi network (155). Interestingly, fibroblasts isolated from these patients revealed abnormalities of actin cytoskeletal assembly (156). These abnormalities are suggested to contribute to the Lowe syndrome phenotypes such as cataract formation. Importantly, PIP2 5 phosphatase binds to the C-terminal RhoGAP domain of Rac GTPase, and activation of Rac results in the translocation of PIP2 5 phosphatase to the cell membrane (155). Thus, the OCRL1 product is believed to be involved in Rac GTPase regulated cell migration and cell adhesions in various cell types.
Myotonic dystrophy kinase, a serine-threonine kinase related to Rho kinase, is expressed in both lens epithelial cells and lens fibers (157). Over expression of myotonic dystrophy kinase in lens epithelial cells induces actin cytoskeletal reorganization and focal adhesion formation similar to RhoA-induced changes, indicating a role for the former kinase in regulation of lens cytoskeletal organization (157). Interestingly, Myotonic dystrophy (DM) is an autosomal dominant disorder characterized by skeletal muscle wasting, cardiac arrhythmia, mental retardation and cataracts (158). The genetic defect in DM is a CTG repeat expansion located in the 3' untranslated region of myotonic dystrophy kinase gene and in the 5' region of a second, homeodomain-encoding gene. Partial loss of function of both these genes is thought to be involved in the DM syndrome (158).
The non-canonical Wnt signaling pathway, in which Wnt activates the Rho family of GTPases, is essential for cell polarity and movements during vertebrate gastrulation. The Wnt pathway shares components with other signaling pathways involved in the establishment of planar cell polarity (PCP) in Drosophila, thus it is often referred to as the Wnt/PCP pathway (129, 130, 159). Wnt proteins activate both RhoA and Rac and their down stream effectors Rho kinase and PAK (130, 160, 161). The localization of Wnts and their Frizzled (Fz) receptors has been confirmed in lens tissues and these proteins are believed to play a role in lens morphogenesis and differentiation (6, 120, 162). Importantly, mouse embryos homozygous for a mutation in the lrp6 gene, which encodes the co-receptor for Wnt signaling, fail to form a normal lens epithelium (120). Smith et al (163) also provided experimental evidence for the involvement of Wnt/Beta-catenin in lens fate and development. Moreover, it has been shown in lens epithelial cultures, that Wnt activates FGF-dependent fiber cell differentiation markers and morphological changes suggesting the importance of Wnt in lens differentiation (121). It is reasonable to speculate that Rho GTPase-mediated signaling through the Wnt/Frizzled/PCP pathways participates in lens fiber cell elongation and differentiation via reorganization of actin cytoskeleton and microtubule network.
Recently Griep et al have also demonstrated that the ocular lens expresses various PDZ domain-containing proteins such as Dlg-1 and Scrib and that these proteins localize to the cell adhesion sites and cell junctional sites in the lens indicating potential involvement in establishment of cell polarity and migration (119, 164). Further, activity of the PDZ-domain proteins has been shown to be critical for lens epithelial cell proliferation and differentiation. Interestingly, the guanine nucleotide exchange factor of Rho GTPases, PDZ-RhoGEF, regulates cell migration, cell adhesion and actin polymerization by activating Rho and Rac GTPases in different cell types (52). Therefore, it is very likely that the signaling pathways involving the PDZ-domain proteins may play an influential role in lens fiber cell elongation, migration and fiber cell-cell interactions.
Concluding remarks
External cues which induce lens epithelial differentiation have been identified in both the anterior and posterior chambers of the eye, and these observations led to the realization that several of the identified growth factors play a fundamental role in lens growth, development and function/integrity in vivo. The transition of a lens epithelial cell to a mature fiber cell is a complex, highly coordinated and multi-step process. While we have a fairly good understanding of how lens epithelial cells exit from cell cycle and initiate transcriptional activity of differentiation-specific genes, our knowledge regarding regulation of elongation, cell shape changes, migration and polarization has lagged behind. Although many of these processes are believed to be influenced by growth factors in the lens, the exact identity of cellular mechanisms that act down stream to external cues are not known. Cell migration, cell shape changes, polarization and cell-cell interactions are regulated fundamentally through actin cytoskeletal dynamics, integrin–mediated cell adhesive interactions, adherens junctions and intracellular signaling mechanisms. Recent advancements in understanding of actin cytoskeletal biology has identified the Rho GTPases (Rho, Rac and Cdc42) as critical regulators of actin cytoskeletal reorganization, contraction, cell adhesions, cell migration, polarity and microtubule assembly. In lens epithelial cells, growth factor stimulated activation of Rho GTPases elicits actin cytoskeletal reorganization, contraction, formation of focal adhesions and cell-cell junctions. Furthermore, inactivation of RhoGTPases in the developing lens disrupts fiber cell elongation and differentiation in association with abnormal fiber cell migration, fiber cell organization and actin cytoskeletal organization, demonstrating a critical role for these proteins in lens fiber cell elongation and differentiation. Myosin II, which interacts with actin to form an actomyosin contractile network, is found to be preferentially activated in elongating and differentiating lens fibers through calcium-calmodulin-dependent myosin light chain kinase and calcium-independent Rho kinase activity. Lens fiber cell migration also critically depends on the c-abl kinase interacting proteins Abi-1 and Abi-2 which regulate actin dynamics via Rac GTPase. Conversely, the polymerization status of actin and its reorganization at the cell cortical regions appears to influence lens differentiation. These recent observations, when viewed in light of earlier findings of increased actin filament assembly being associated with lens fiber cell elongation, and the lens fiber-specific distribution of actin capping proteins such as tropomodulin, indicate the pivotal significance of actin cytoskeletal dynamics as an important mechanism regulating lens differentiation. The Rho GTPases are believed to be involved in every cell process that exhibits a dependence on organization of the actin cytoskeleton and microtubule dynamics. Importantly, given how the activity of the Rho GTPases is fine-tuned by several upstream and downstream effector proteins, a detailed understanding of the distribution pattern of such effector proteins is necessary for a better appreciation of the role played by the RhoGTPases in lens fiber cell elongation and differentiation. Importantly, there is complex cross talk between Rho GTPase-dependent pathways and other signaling pathways that regulate actin dynamics and cell adhesions. Therefore, it is necessary to utilize both mechanistic and genetic approaches to unravel the relative significance of any single signaling pathway. Conditional knock outs and siRNA techniques are promising approaches that could be applied to evaluate these hypotheses.
Acknowledgments
Supported by NIH/NEI grants EY013573, EY12201 (PVR), P-30 EY05722 and funding from the Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–53. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 2.Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74(1):1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- 3.Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–96. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- 4.Kuszak JR, Zoltoski RK, Tiedemann CE. Development of lens sutures. Int J Dev Biol. 2004;48(8–9):889–902. doi: 10.1387/ijdb.041880jk. [DOI] [PubMed] [Google Scholar]
- 5.McAvoy JW, Chamberlain CG, de Iongh RU, Hales AM, Lovicu FJ. Lens development. Eye. 1999;13 (Pt 3b):425–37. doi: 10.1038/eye.1999.117. [DOI] [PubMed] [Google Scholar]
- 6.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280(1):1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Taylor VL, al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, Costello MJ. Morphology of the normal human lens. Invest Ophthalmol Vis Sci. 1996;37(7):1396–410. [PubMed] [Google Scholar]
- 8.Piatigorsky J. Lens cell elongation in vitro and microtubules. Ann N Y Acad Sci. 1975;253:333–47. doi: 10.1111/j.1749-6632.1975.tb19211.x. [DOI] [PubMed] [Google Scholar]
- 9.Bassnett S, Missey H, Vucemilo I. Molecular architecture of the lens fiber cell basal membrane complex. J Cell Sci. 1999;112 (Pt 13):2155–65. doi: 10.1242/jcs.112.13.2155. [DOI] [PubMed] [Google Scholar]
- 10.Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest Ophthalmol Vis Sci. 2001;42(3):727–34. [PubMed] [Google Scholar]
- 11.Zelenka PS. Regulation of cell adhesion and migration in lens development. Int J Dev Biol. 2004;48(8–9):857–65. doi: 10.1387/ijdb.041871pz. [DOI] [PubMed] [Google Scholar]
- 12.Menko AS. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–90. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- 13.Lee A, Fischer RS, Fowler VM. Stabilization and remodeling of the membrane skeleton during lens fiber cell differentiation and maturation. Dev Dyn. 2000;217(3):257–70. doi: 10.1002/(SICI)1097-0177(200003)217:3<257::AID-DVDY4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Bloemendal H, Berbers GA, De Jong WW, et al. Interaction of crystallins with the cytoskeletal-plasma membrane complex of the bovine lens. Ciba Found Symp. 1984;106:177–90. doi: 10.1002/9780470720875.ch10. [DOI] [PubMed] [Google Scholar]
- 15.Perng MD, Quinlan RA. Seeing is believing! The optical properties of the eye lens are dependent upon a functional intermediate filament cytoskeleton. Exp Cell Res. 2005;305(1):1–9. doi: 10.1016/j.yexcr.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: many roles for Pax-6. Bioessays. 1996;18(8):621–30. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- 17.Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol Vis. 2003;9:329–36. [PubMed] [Google Scholar]
- 18.Maddala R, Deng PF, Costello JM, Wawrousek EF, Zigler JS, Rao VP. Impaired cytoskeletal organization and membrane integrity in lens fibers of a Rho GTPase functional knockout transgenic mouse. Lab Invest. 2004;84(6):679–92. doi: 10.1038/labinvest.3700105. [DOI] [PubMed] [Google Scholar]
- 19.Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol. 2006;295(2):714–29. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 20.Clark JI, Matsushima H, David LL, Clark JM. Lens cytoskeleton and transparency: a model. Eye. 1999;13 (Pt 3b):417–24. doi: 10.1038/eye.1999.116. [DOI] [PubMed] [Google Scholar]
- 21.Quinlan RA, Sandilands A, Procter JE, et al. The eye lens cytoskeleton. Eye. 1999;13 (Pt 3b):409–16. doi: 10.1038/eye.1999.115. [DOI] [PubMed] [Google Scholar]
- 22.Tardieu A. Eye lens proteins and transparency: from light transmission theory to solution X-ray structural analysis. Annu Rev Biophys Biophys Chem. 1988;17:47–70. doi: 10.1146/annurev.bb.17.060188.000403. [DOI] [PubMed] [Google Scholar]
- 23.Kilic F, Trevithick JR. Vitamin C reduces cytochalasin D cataractogenesis. Curr Eye Res. 1995;14(10):943–9. doi: 10.3109/02713689508995134. [DOI] [PubMed] [Google Scholar]
- 24.Mikuni I, Fujiwara T, Obazawa H. Microtubules in experimental cataracts: disappearance of microtubules of epithelial cells and lens fibers in colchicine-induced cataracts. Tokai J Exp Clin Med. 1981;6(3):297–303. [PubMed] [Google Scholar]
- 25.Ireland M, Lieska N, Maisel H. Lens actin: purification and localization. Exp Eye Res. 1983;37(4):393–408. doi: 10.1016/0014-4835(83)90176-8. [DOI] [PubMed] [Google Scholar]
- 26.Kibbelaar MA, Selten-Versteegen AM, Dunia I, Benedetti EL, Bloemendal H. Actin in mammalian lens. Eur J Biochem. 1979;95(3):543–9. doi: 10.1111/j.1432-1033.1979.tb12995.x. [DOI] [PubMed] [Google Scholar]
- 27.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 28.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 29.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 30.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33(Pt 5):891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 31.Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Curr Opin Cell Biol. 1999;11(2):274–86. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 32.Rafferty NS, Scholz DL. Actin in polygonal arrays of microfilaments and sequestered actin bundles (SABs) in lens epithelial cells of rabbits and mice. Curr Eye Res. 1985;4(6):713–8. doi: 10.3109/02713688509017667. [DOI] [PubMed] [Google Scholar]
- 33.Rafferty NS, Scholz DL. Development of actin polygonal arrays in rabbit lens epithelial cells. Curr Eye Res. 1991;10(7):637–43. doi: 10.3109/02713689109013855. [DOI] [PubMed] [Google Scholar]
- 34.Rafferty NS, Scholz DL, Goldberg M, Lewyckyj M. Immunocytochemical evidence for an actin-myosin system in lens epithelial cells. Exp Eye Res. 1990;51(5):591–600. doi: 10.1016/0014-4835(90)90090-h. [DOI] [PubMed] [Google Scholar]
- 35.Ramaekers FC, Poels LG, Jap PH, Bloemendal H. Simultaneous demonstration of microfilaments and intermediate-sized filaments in the lens by double immunofluorescence. Exp Eye Res. 1982;35(4):363–9. doi: 10.1016/0014-4835(82)90099-9. [DOI] [PubMed] [Google Scholar]
- 36.Kibbelaar MA, Ramaekers FC, Ringens PJ, et al. Is actin in eye lens a possible factor in visual accomodation? Nature. 1980;285(5765):506–8. doi: 10.1038/285506a0. [DOI] [PubMed] [Google Scholar]
- 37.Arruti C, Courtois Y. Morphological changes and growth stimulation of bovine epithelial lens cells by a retinal extract in vitro. Exp Cell Res. 1978;117(2):283–92. doi: 10.1016/0014-4827(78)90142-8. [DOI] [PubMed] [Google Scholar]
- 38.Courtois Y, Arruti C, Barritault D, Tassin J, Olivie M, Hughes RC. Modulation of the shape of epithelial lens cells in vitro directed by a retinal extract factor. A model of interconversions and the role of actin filaments and fibronectin. Differentiation. 1981;18(1):11–27. doi: 10.1111/j.1432-0436.1981.tb01100.x. [DOI] [PubMed] [Google Scholar]
- 39.Ramaekers FC, Boomkens TR, Bloemendal H. Cytoskeletal and contractile structures in bovine lens cell differentiation. Exp Cell Res. 1981;135(2):454–61. doi: 10.1016/0014-4827(81)90190-7. [DOI] [PubMed] [Google Scholar]
- 40.Ramaekers F, Jap P, Mungyer G, Bloemendal H. Microfilament assembly during lens cell elongation in vitro. Curr Eye Res. 1982;2(3):169–81. doi: 10.3109/02713688208997691. [DOI] [PubMed] [Google Scholar]
- 41.Maisel H, Atreya P. N-cadherin detected in the membrane fraction of lens fiber cells. Experientia. 1990;46(2):222–3. doi: 10.1007/BF02027322. [DOI] [PubMed] [Google Scholar]
- 42.Bagchi M, Katar M, Lewis J, Maisel H. Associated proteins of lens adherens junction. J Cell Biochem. 2002;86(4):700–3. doi: 10.1002/jcb.10258. [DOI] [PubMed] [Google Scholar]
- 43.Bagchi M, Katar M, Lo WK, Yost R, Hill C, Maisel H. ERM proteins of the lens. J Cell Biochem. 2004;92(3):626–30. doi: 10.1002/jcb.20062. [DOI] [PubMed] [Google Scholar]
- 44.Mousa GY, Trevithick JR. Actin in the lens: changes in actin during differentiation of lens epithelial cells in vivo. Exp Eye Res. 1979;29(1):71–81. doi: 10.1016/0014-4835(79)90167-2. [DOI] [PubMed] [Google Scholar]
- 45.Beebe DC, Cerrelli S. Cytochalasin prevents cell elongation and increases potassium efflux from embryonic lens epithelial cells: implications for the mechanism of lens fiber cell elongation. Lens Eye Toxic Res. 1989;6(4):589–601. [PubMed] [Google Scholar]
- 46.Mousa GY, Trevithick JR. Differentiation of rat lens epithelial cells in tissue culture. II. Effects of cytochalasins B and D on actin organization and differentiation. Dev Biol. 1977;60(1):14–25. doi: 10.1016/0012-1606(77)90107-5. [DOI] [PubMed] [Google Scholar]
- 47.Iwig M, Glaesser D, Bethge M. Cell shape-mediated growth control of lens epithelial cells grown in culture. Exp Cell Res. 1981;131(1):47–55. doi: 10.1016/0014-4827(81)90404-3. [DOI] [PubMed] [Google Scholar]
- 48.Iwig M, Glasser D. [Participation of microfilaments in the formation of adhesion plaques and their importance in cell shape and proliferative regulation] Acta Histochem Suppl. 1990;39:403–18. [PubMed] [Google Scholar]
- 49.Menko S, Philp N, Veneziale B, Walker J. Integrins and development: how might these receptors regulate differentiation of the lens. Ann N Y Acad Sci. 1998;842:36–41. doi: 10.1111/j.1749-6632.1998.tb09629.x. [DOI] [PubMed] [Google Scholar]
- 50.Ferreira-Cornwell MC, Veneziale RW, Grunwald GB, Menko AS. N-cadherin function is required for differentiation-dependent cytoskeletal reorganization in lens cells in vitro. Exp Cell Res. 2000;256(1):237–47. doi: 10.1006/excr.2000.4819. [DOI] [PubMed] [Google Scholar]
- 51.Walker JL, Menko AS. alpha6 Integrin is regulated with lens cell differentiation by linkage to the cytoskeleton and isoform switching. Dev Biol. 1999;210(2):497–511. doi: 10.1006/dbio.1999.9277. [DOI] [PubMed] [Google Scholar]
- 52.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 53.Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol. 1999;11(1):26–33. doi: 10.1016/s0955-0674(99)80004-0. [DOI] [PubMed] [Google Scholar]
- 54.Sellers JR. Myosins: a diverse superfamily. Biochim Biophys Acta. 2000;1496(1):3–22. doi: 10.1016/s0167-4889(00)00005-7. [DOI] [PubMed] [Google Scholar]
- 55.Ireland M, Maisel H. Identification of native actin filaments in chick lens fiber cells. Exp Eye Res. 1983;36(4):531–6. doi: 10.1016/0014-4835(83)90046-5. [DOI] [PubMed] [Google Scholar]
- 56.Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore) 2003;82(3):203–15. doi: 10.1097/01.md.0000076006.64510.5c. [DOI] [PubMed] [Google Scholar]
- 57.Woo MK, Fowler VM. Identification and characterization of tropomodulin and tropomyosin in the adult rat lens. J Cell Sci. 1994;107 (Pt 5):1359–67. doi: 10.1242/jcs.107.5.1359. [DOI] [PubMed] [Google Scholar]
- 58.Fischer RS, Lee A, Fowler VM. Tropomodulin and tropomyosin mediate lens cell actin cytoskeleton reorganization in vitro. Invest Ophthalmol Vis Sci. 2000;41(1):166–74. [PubMed] [Google Scholar]
- 59.Sussman MA, McAvoy JW, Rudisill M, et al. Lens tropomodulin: developmental expression during differentiation. Exp Eye Res. 1996;63(2):223–32. doi: 10.1006/exer.1996.0111. [DOI] [PubMed] [Google Scholar]
- 60.Barber MA, Welch HC. PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer. 2006;93(5):E44–52. [PubMed] [Google Scholar]
- 61.Webb DJ, Zhang H, Horwitz AF. Cell migration: an overview. Methods Mol Biol. 2005;294:3–11. [PubMed] [Google Scholar]
- 62.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265(1):23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Small JV, Resch GP. The comings and goings of actin: coupling protrusion and retraction in cell motility. Curr Opin Cell Biol. 2005;17(5):517–23. doi: 10.1016/j.ceb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 64.Rao PV, Zigler JS, Jr, Garland D. Analysis of small GTP-binding proteins of the lens by GTP overlay assay reveals the presence of unique GTP-binding proteins associated with fiber cells. Exp Eye Res. 1997;64(2):219–27. doi: 10.1006/exer.1996.0197. [DOI] [PubMed] [Google Scholar]
- 65.Maddala R, Peng YW, Rao PV. Selective expression of the small GTPase RhoB in the early developing mouse lens. Dev Dyn. 2001;222(3):534–7. doi: 10.1002/dvdy.1202. [DOI] [PubMed] [Google Scholar]
- 66.Lehto VP, Virtanen I. Vinculin in cultured bovine lens-forming cells. Cell Differ. 1985;16(3):153–60. doi: 10.1016/0045-6039(85)90512-3. [DOI] [PubMed] [Google Scholar]
- 67.Geiger B, Volk T, Volberg T. Molecular heterogeneity of adherens junctions. J Cell Biol. 1985;101(4):1523–31. doi: 10.1083/jcb.101.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maddala R, Rao VP. alpha-Crystallin localizes to the leading edges of migrating lens epithelial cells. Exp Cell Res. 2005;306(1):203–15. doi: 10.1016/j.yexcr.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 69.Straub BK, Boda J, Kuhn C, et al. A novel cell-cell junction system: the cortex adhaerens mosaic of lens fiber cells. J Cell Sci. 2003;116(Pt 24):4985–95. doi: 10.1242/jcs.00815. [DOI] [PubMed] [Google Scholar]
- 70.Iwig M, Glaesser D. Cell-substratum interactions and the cytoskeleton in cell shape-mediated growth regulation of lens epithelial cells. Lens Eye Toxic Res. 1991;8(2–3):281–309. [PubMed] [Google Scholar]
- 71.Blanquet PR, Courtois Y. Differential assemblage of the basal membrane-cytoskeleton complex in bovine epithelial lens cells. Exp Eye Res. 1989;48(2):187–207. doi: 10.1016/s0014-4835(89)80069-7. [DOI] [PubMed] [Google Scholar]
- 72.Al-Ghoul KJ, Kuszak JR, Lu JY, Owens MJ. Morphology and organization of posterior fiber ends during migration. Mol Vis. 2003;9:119–28. [PubMed] [Google Scholar]
- 73.Grove M, Demyanenko G, Echarri A, et al. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol. 2004;24(24):10905–22. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stradal T, Courtney KD, Rottner K, Hahne P, Small JV, Pendergast AM. The Abl interactor proteins localize to sites of actin polymerization at the tips of lamellipodia and filopodia. Curr Biol. 2001;11(11):891–5. doi: 10.1016/s0960-9822(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 75.Leng Y, Zhang J, Badour K, et al. Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for WAVE2 activation. Proc Natl Acad Sci U S A. 2005;102(4):1098–103. doi: 10.1073/pnas.0409120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stradal TE, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14(6):303–11. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Stuart JR, Gonzalez FH, Kawai H, Yuan ZM. C-ABL interacts with the wave2 signaling complex to induce membrane ruffling and cell spreading. J Biol Chem. 2006 doi: 10.1074/jbc.M602389200. [DOI] [PubMed] [Google Scholar]
- 78.Miki H, Takenawa T. Regulation of actin dynamics by WASP family proteins. J Biochem (Tokyo) 2003;134(3):309–13. doi: 10.1093/jb/mvg146. [DOI] [PubMed] [Google Scholar]
- 79.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 80.Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13(5):584–92. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 81.Menko AS, Philip NJ. Beta 1 integrins in epithelial tissues: a unique distribution in the lens. Exp Cell Res. 1995;218(2):516–21. doi: 10.1006/excr.1995.1186. [DOI] [PubMed] [Google Scholar]
- 82.Wederell ED, Brown H, O'Connor M, Chamberlain CG, McAvoy JW, de Iongh RU. Laminin-binding integrins in rat lens morphogenesis and their regulation during fibre differentiation. Exp Eye Res. 2005;81(3):326–39. doi: 10.1016/j.exer.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 83.Walker JL, Zhang L, Menko AS. A signaling role for the uncleaved form of alpha 6 integrin in differentiating lens fiber cells. Dev Biol. 2002;251(2):195–205. doi: 10.1006/dbio.2002.0823. [DOI] [PubMed] [Google Scholar]
- 84.Lim JM, Kim JA, Lee JH, Joo CK. Downregulated expression of integrin alpha6 by transforming growth factor-beta(1) on lens epithelial cells in vitro. Biochem Biophys Res Commun. 2001;284(1):33–41. doi: 10.1006/bbrc.2001.4942. [DOI] [PubMed] [Google Scholar]
- 85.Duncan MK, Kozmik Z, Cveklova K, Piatigorsky J, Cvekl A. Overexpression of PAX6(5a) in lens fiber cells results in cataract and upregulation of (alpha)5(beta)1 integrin expression. J Cell Sci. 2000;113 (Pt 18):3173–85. doi: 10.1242/jcs.113.18.3173. [DOI] [PubMed] [Google Scholar]
- 86.Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat Rev Mol Cell Biol. 2001;2(12):887–97. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- 87.Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res. 2002;35(2):239–46. doi: 10.4067/s0716-97602002000200016. [DOI] [PubMed] [Google Scholar]
- 88.Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004;14(11):589–93. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 89.Leong L, Menko AS, Grunwald GB. Differential expression of N- and B-cadherin during lens development. Invest Ophthalmol Vis Sci. 2000;41(11):3503–10. [PubMed] [Google Scholar]
- 90.Citi S, Volberg T, Bershadsky AD, Denisenko N, Geiger B. Cytoskeletal involvement in the modulation of cell-cell junctions by the protein kinase inhibitor H-7. J Cell Sci. 1994;107 (Pt 3):683–92. [PubMed] [Google Scholar]
- 91.Volk T, Geiger B. A-CAM: a 135-kD receptor of intercellular adherens junctions. I. Immunoelectron microscopic localization and biochemical studies. J Cell Biol. 1986;103(4):1441–50. doi: 10.1083/jcb.103.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Volk T, Cohen O, Geiger B. Formation of heterotypic adherens-type junctions between L-CAM-containing liver cells and A-CAM-containing lens cells. Cell. 1987;50(6):987–94. doi: 10.1016/0092-8674(87)90525-3. [DOI] [PubMed] [Google Scholar]
- 93.Maddala RL, Reddy VN, Rao PV. Lovastatin-induced cytoskeletal reorganization in lens epithelial cells: role of Rho GTPases. Invest Ophthalmol Vis Sci. 2001;42(11):2610–5. [PubMed] [Google Scholar]
- 94.Lo WK. Adherens junctions in the ocular lens of various species: ultrastructural analysis with an improved fixation. Cell Tissue Res. 1988;254(1):31–40. doi: 10.1007/BF00220014. [DOI] [PubMed] [Google Scholar]
- 95.More MI, Kirsch FP, Rathjen FG. Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers leading to cataract formation. J Cell Biol. 2001;154(1):187–96. doi: 10.1083/jcb.200104038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walker JL, Zhang L, Menko AS. Transition between proliferation and differentiation for lens epithelial cells is regulated by Src family kinases. Dev Dyn. 2002;224(4):361–72. doi: 10.1002/dvdy.10115. [DOI] [PubMed] [Google Scholar]
- 97.Louvet-Vallee S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000;92(5):305–16. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- 98.Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112(2):165–76. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gautreau A, Louvard D, Arpin M. ERM proteins and NF2 tumor suppressor: the Yin and Yang of cortical actin organization and cell growth signaling. Curr Opin Cell Biol. 2002;14(1):104–9. doi: 10.1016/s0955-0674(01)00300-3. [DOI] [PubMed] [Google Scholar]
- 100.Manchanda N, Lyubimova A, Ho HY, et al. The NF2 tumor suppressor Merlin and the ERM proteins interact with N-WASP and regulate its actin polymerization function. J Biol Chem. 2005;280(13):12517–22. doi: 10.1074/jbc.C400583200. [DOI] [PubMed] [Google Scholar]
- 101.Takahashi K, Sasaki T, Mammoto A, et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272(37):23371–5. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 102.Kaye LD, Rothner AD, Beauchamp GR, Meyers SM, Estes ML. Ocular findings associated with neurofibromatosis type II. Ophthalmology. 1992;99(9):1424–9. doi: 10.1016/s0161-6420(92)31789-0. [DOI] [PubMed] [Google Scholar]
- 103.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76(2):145–53. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 104.Del Vecchio PJ, MacElroy KS, Rosser MP, Church RL. Association of alpha-crystallin with actin in cultured lens cells. Curr Eye Res. 1984;3(10):1213–9. doi: 10.3109/02713688409000824. [DOI] [PubMed] [Google Scholar]
- 105.Gopalakrishnan S, Takemoto L. Binding of actin to lens alpha crystallins. Curr Eye Res. 1992;11(9):929–33. doi: 10.3109/02713689209033490. [DOI] [PubMed] [Google Scholar]
- 106.Lo WK, Shaw AP, Wen XJ. Actin filament bundles in cortical fiber cells of the rat lens. Exp Eye Res. 1997;65(5):691–701. doi: 10.1006/exer.1997.0375. [DOI] [PubMed] [Google Scholar]
- 107.Lo WK, Mills A, Kuck JF. Actin filament bundles are associated with fiber gap junctions in the primate lens. Exp Eye Res. 1994;58(2):189–96. doi: 10.1006/exer.1994.1007. [DOI] [PubMed] [Google Scholar]
- 108.Lo WK, Shaw AP, Paulsen DF, Mills A. Spatiotemporal distribution of zonulae adherens and associated actin bundles in both epithelium and fiber cells during chicken lens development. Exp Eye Res. 2000;71(1):45–55. doi: 10.1006/exer.2000.0848. [DOI] [PubMed] [Google Scholar]
- 109.Giessmann D, Theiss C, Breipohl W, Meller K. Microinjection of actin antibodies impaired gap junctional intercellular communication in lens epithelial cells in vitro. Curr Eye Res. 2003;27(3):157–64. doi: 10.1076/ceyr.27.3.157.16054. [DOI] [PubMed] [Google Scholar]
- 110.Zhou CJ, Lo WK. Association of clathrin, AP-2 adaptor and actin cytoskeleton with developing interlocking membrane domains of lens fibre cells. Exp Eye Res. 2003;77(4):423–32. doi: 10.1016/s0014-4835(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 111.Kilic F, Trevithick JR. Modelling cortical cataractogenesis. XXIX. Calpain proteolysis of lens fodrin in cataract. Biochem Mol Biol Int. 1998;45(5):963–78. doi: 10.1002/iub.7510450514. [DOI] [PubMed] [Google Scholar]
- 112.Rao V, Wawrousek E, Tamm ER, Zigler S., Jr Rho GTPase inactivation impairs lens growth and integrity. Lab Invest. 2002;82(2):231–9. doi: 10.1038/labinvest.3780415. [DOI] [PubMed] [Google Scholar]
- 113.Rao PV, Robison WG, Jr, Bettelheim F, Lin LR, Reddy VN, Zigler JS., Jr Role of small GTP-binding proteins in lovastatin-induced cataracts. Invest Ophthalmol Vis Sci. 1997;38(11):2313–21. [PubMed] [Google Scholar]
- 114.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 115.Ridley AJ. Rho GTPases and cell migration. J Cell Sci. 2001;114(Pt 15):2713–22. doi: 10.1242/jcs.114.15.2713. [DOI] [PubMed] [Google Scholar]
- 116.Duncan G, Collison DJ. Calcium signalling in ocular tissues: functional activity of G-protein and tyrosine-kinase coupled receptors. Exp Eye Res. 2002;75(4):377–89. [PubMed] [Google Scholar]
- 117.Chandrasekher G, Sailaja D. Differential activation of phosphatidylinositol 3-kinase signaling during proliferation and differentiation of lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44(10):4400–11. doi: 10.1167/iovs.03-0136. [DOI] [PubMed] [Google Scholar]
- 118.Negash S, Wang HS, Gao C, Ledee D, Zelenka P. Cdk5 regulates cell-matrix and cell-cell adhesion in lens epithelial cells. J Cell Sci. 2002;115(Pt 10):2109–17. doi: 10.1242/jcs.115.10.2109. [DOI] [PubMed] [Google Scholar]
- 119.Nguyen MM, Nguyen ML, Caruana G, Bernstein A, Lambert PF, Griep AE. Requirement of PDZ-containing proteins for cell cycle regulation and differentiation in the mouse lens epithelium. Mol Cell Biol. 2003;23(24):8970–81. doi: 10.1128/MCB.23.24.8970-8981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stump RJ, Ang S, Chen Y, et al. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259(1):48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- 121.Lyu J, Joo CK. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development. 2004;131(8):1813–24. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- 122.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–86. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 123.Narumiya S, Yasuda S. Rho GTPases in animal cell mitosis. Curr Opin Cell Biol. 2006;18(2):199–205. doi: 10.1016/j.ceb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–81. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 125.Daniels RH, Bokoch GM. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem Sci. 1999;24(9):350–5. doi: 10.1016/s0968-0004(99)01442-5. [DOI] [PubMed] [Google Scholar]
- 126.Treisman R, Alberts AS, Sahai E. Regulation of SRF activity by Rho family GTPases. Cold Spring Harb Symp Quant Biol. 1998;63:643–51. doi: 10.1101/sqb.1998.63.643. [DOI] [PubMed] [Google Scholar]
- 127.Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15(5):590–7. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 128.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85(4):1159–204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 129.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 130.Habas R, He X. Activation of Rho and Rac by Wnt/frizzled signaling. Methods Enzymol. 2006;406:500–11. doi: 10.1016/S0076-6879(06)06038-1. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y, Stump RJW, Lovicu FJ, McAvoy JW. A role for Wnt/Planar Cell polarity signaling during lens fiber cell differentiation? Seminars in Cell and Developmental Biology. 2006 doi: 10.1016/j.semcdb.2006.11.005. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Winter-Vann AM, Casey PJ. Post-prenylation-processing enzymes as new targets in oncogenesis. Nat Rev Cancer. 2005;5(5):405–12. doi: 10.1038/nrc1612. [DOI] [PubMed] [Google Scholar]
- 133.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cheng Q, Gerald Robison W, Samuel Zigler J. Geranylgeranyl pyrophosphate counteracts the cataractogenic effect of lovastatin on cultured rat lenses. Exp Eye Res. 2002;75(5):603–9. doi: 10.1006/exer.2002.2053. [DOI] [PubMed] [Google Scholar]
- 135.Girao H, Pereira P, Ramalho J, Quinlan R, Prescott A. Cholesterol oxides mediated changes in cytoskeletal organisation involves Rho GTPases small star, filled. Exp Cell Res. 2003;291(2):502–13. doi: 10.1016/j.yexcr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 136.Hoffmann G, Gibson KM, Brandt IK, Bader PI, Wappner RS, Sweetman L. Mevalonic aciduria--an inborn error of cholesterol and nonsterol isoprene biosynthesis. N Engl J Med. 1986;314(25):1610–4. doi: 10.1056/NEJM198606193142504. [DOI] [PubMed] [Google Scholar]
- 137.Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48(8–9):783–91. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- 138.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26(8):870–81. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 139.Pages C, Simon MF, Valet P, Saulnier-Blache JS. Lysophosphatidic acid synthesis and release. Prostaglandins Other Lipid Mediat. 2001;64(1–4):1–10. doi: 10.1016/s0090-6980(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 140.Maddala R, Reddy VN, Rao PV. Lysophospholipid growth factors and Edg receptor-mediated signaling in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:Abstract 1256. [Google Scholar]
- 141.James C, Collison DJ, Duncan G. Characterization and functional activity of thrombin receptors in the human lens. Invest Ophthalmol Vis Sci. 2005;46(3):925–32. doi: 10.1167/iovs.04-0523. [DOI] [PubMed] [Google Scholar]
- 142.Maddala R, Qiu J, Rao PV. Regulation of myosin light chain phosphorylation in lens epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:1711. [Google Scholar]
- 143.de Iongh RU, Lovicu FJ, Overbeek PA, et al. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development. 2001;128(20):3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- 144.Wormstone IM, Tamiya S, Anderson I, Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002;43(7):2301–8. [PubMed] [Google Scholar]
- 145.Zatechka SD, Jr, Lou MF. Studies of the mitogen-activated protein kinases and phosphatidylinositol-3 kinase in the lens. 1. The mitogenic and stress responses. Exp Eye Res. 2002;74(6):703–17. doi: 10.1006/exer.2002.1168. [DOI] [PubMed] [Google Scholar]
- 146.Ridley AJ. Rho proteins, PI 3-kinases, and monocyte/macrophage motility. FEBS Lett. 2001;498(2–3):168–71. doi: 10.1016/s0014-5793(01)02481-4. [DOI] [PubMed] [Google Scholar]
- 147.Rafferty NS, Rafferty KA, Ito E. Agonist-induced rise in intracellular calcium of lens epithelial cells: effects on the actin cytoskeleton. Exp Eye Res. 1994;59(2):191–201. doi: 10.1006/exer.1994.1097. [DOI] [PubMed] [Google Scholar]
- 148.Wagner LM, Fowler VM, Takemoto DJ. The interaction and phosphorylation of tropomodulin by protein kinase Calpha in N/N 1003A lens epithelial cells. Mol Vis. 2002;8:394–406. [PubMed] [Google Scholar]
- 149.Steffen A, Rottner K, Ehinger J, et al. Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. Embo J. 2004;23(4):749–59. doi: 10.1038/sj.emboj.7600084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fan PD, Goff SP. Abl interactor 1 binds to sos and inhibits epidermal growth factor- and v-Abl-induced activation of extracellular signal-regulated kinases. Mol Cell Biol. 2000;20(20):7591–601. doi: 10.1128/mcb.20.20.7591-7601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smith D. Cdk5 in neuroskeletal dynamics. Neurosignals. 2003;12(4–5):239–51. doi: 10.1159/000074626. [DOI] [PubMed] [Google Scholar]
- 152.Ledee DR, Gao CY, Seth R, Fariss RN, Tripathi BK, Zelenka PS. A specific interaction between muskelin and the cyclin-dependent kinase 5 activator p39 promotes peripheral localization of muskelin. J Biol Chem. 2005;280(22):21376–83. doi: 10.1074/jbc.M501215200. [DOI] [PubMed] [Google Scholar]
- 153.Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395(6698):194–8. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- 154.Tripathi RC, Cibis GW, Tripathi BJ. Pathogenesis of cataracts in patients with Lowe's syndrome. Ophthalmology. 1986;93(8):1046–51. doi: 10.1016/s0161-6420(86)33622-4. [DOI] [PubMed] [Google Scholar]
- 155.Faucherre A, Desbois P, Nagano F, et al. Lowe syndrome protein Ocrl1 is translocated to membrane ruffles upon Rac GTPase activation: a new perspective on Lowe syndrome pathophysiology. Hum Mol Genet. 2005;14(11):1441–8. doi: 10.1093/hmg/ddi153. [DOI] [PubMed] [Google Scholar]
- 156.Suchy SF, Nussbaum RL. The deficiency of PIP2 5-phosphatase in Lowe syndrome affects actin polymerization. Am J Hum Genet. 2002;71(6):1420–7. doi: 10.1086/344517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jin S, Shimizu M, Balasubramanyam A, Epstein HF. Myotonic dystrophy protein kinase (DMPK) induces actin cytoskeletal reorganization and apoptotic-like blebbing in lens cells. Cell Motil Cytoskeleton. 2000;45(2):133–48. doi: 10.1002/(SICI)1097-0169(200002)45:2<133::AID-CM5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 158.Klesert TR, Cho DH, Clark JI, et al. Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat Genet. 2000;25(1):105–9. doi: 10.1038/75490. [DOI] [PubMed] [Google Scholar]
- 159.Barrow JR. Wnt/PCP signaling: A veritable polar star in establishing patterns of polarity in embryonic tissues. Semin Cell Dev Biol. 2006;17(2):185–93. doi: 10.1016/j.semcdb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 160.Zhu S, Liu L, Korzh V, Gong Z, Low BC. RhoA acts downstream of Wnt5 and Wnt11 to regulate convergence and extension movements by involving effectors Rho kinase and Diaphanous: use of zebrafish as an in vivo model for GTPase signaling. Cell Signal. 2006;18(3):359–72. doi: 10.1016/j.cellsig.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 161.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8(1):34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 162.Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. Expression of Frizzleds and secreted frizzled-related proteins (Sfrps) during mammalian lens development. Int J Dev Biol. 2004;48(8–9):867–77. doi: 10.1387/ijdb.041882yc. [DOI] [PubMed] [Google Scholar]
- 163.Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285(2):477–89. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 164.Nguyen MM, Rivera C, Griep AE. Localization of PDZ domain containing proteins Discs Large-1 and Scribble in the mouse eye. Mol Vis. 2005;11:1183–99. [PubMed] [Google Scholar]


