Abstract
Lymphoid Enhancer Factor 1 (Lef-1) is an important developmental transcription factor required for the inductive formation of several epithelial-derived organs including hair follicles. Inductive expression of Lef-1 mRNA is tightly regulated during embryo development, suggesting the involvement of a highly regulated promoter. In vitro analysis of the Lef-1 gene has demonstrated the existence of at least two spatially distinct promoters with multiple transcriptional start sites that are responsive to the canonical Wnt/β-catenin pathway. Regions of the Lef-1 promoter required for inductive regulation in vivo, however, have yet to be determined. To this end, we utilized LacZ-reporter transgenic mice to define segments of the human Lef-1 promoter capable of reproducing mesenchymal- or epithelial-restricted transcriptional patterns of Lef-1 expression during hair and vibrissa follicle development. These studies have revealed that a 110 bp Wnt/β-catenin-responsive element, contained within a minimal 2.5 kb Lef-1 promoter, plays an important role in regulating mesenchymal, and potentially epithelial, expression during follicle development in mouse embryos. This 2.5 kb Lef-1 promoter also demonstrated inductive mesenchymal expression during postnatal anagen stage hair-follicle cycling. Additionally, analysis of Lef-1 promoter expression revealed previously uncharacterized regions of endogenous Lef-1 expression seen in the sebaceous glands of vibrissa and hair follicles in transgenic lines harboring the minimal Lef-1 promoter and additional intronic sequences. In summary, these studies have begun to dissect the transcriptional diversity of the human Lef-1 promoter during the hair/vibrissa follicle and sebaceous gland formation.
Keywords: development, hair follicle, Lef-1, promoter, sebaceous gland, transgenic mice
During embryogenesis, the development of numerous organs is controlled by highly regulated interactions between bud-forming epithelia and the underlying me-senchymal layers that stimulate signal transduction cascades and the transcription of genes important to epithelial cell movement and differentiation. The Wnt/β-catenin/Lef-1 (Lymphoid Enhancer Factor 1) signaling pathway is one of the most extensively studied transcriptional cascades involved in various types of organogenesis (Gat et al, 1998; Duan et al, 1999 Galceran et al, 1999; Fuchs et al, 2001; Yasumoto et al, 2002). In this context, Wnt proteins bind to transmembrane Frizzled receptors and activate signaling cascades by stabilizing β-catenin and its interactions with transcription factors of the TCF/Lef-1 family. Following translocation to the nucleus, β-catenin/Lef-1 and β-catenin/TCF complexes modulate transcription of target genes through the assembly of multi-protein complexes (Hsu et al, 1998; Novak et al, 1998; Billin et al, 2000; Brantjes et al, 2001).
The TCF/LEF family is composed of Lef-1, Tcf-1, Tcf-3, and Tcf-4. Studies using Lef-1 knockout models have demonstrated that expression of the Lef-1 gene is required for a wide range of inductive epithelial/mesenchymal interactions involved in mammary gland, tooth, vibrissa, hair, and airway-submucosal-gland development (van Genderen et al, 1994; Duan et al, 1999). During hair-follicle development, Lef-1 mRNA and protein are induced in the follicular ectoderm at the leading edge of the invaginating placode, the follicle bulb, the lower epithelial shaft of adult follicles, and the dermal papilla (Oosterwegel et al, 1993; van Genderen et al, 1994; Zhou et al, 1995).
Evidence for the involvement of Wnt/β-catenin/Lef-1 signaling in the early developmental stages of the pelage hair follicle and postnatal hair cycling has been demonstrated using TOPβ-galactosidase (TOPGAL) reporter assays in transgenic mice. TOPGAL, an artificial promoter optimized for Tcf transcriptional induction, demonstrates reporter-gene expression in the placodes and dermal condensates during hair-follicle development in transgenic mice (DasGupta and Fuchs, 1999). These studies utilizing Lef-1 transcriptional reporters have been useful in locating functional β-catenin/Lef-1 complexes at their sites of induction during development. Recently, several studies have also begun to dissect the signals responsible for regulating the Lef-1 gene. In vitro studies dissecting the human Lef-1 promoter have demonstrated transcriptional responsiveness to Wnt3A and β-catenin (Filali et al, 2002). Other studies have demonstrated that isolated segments of the promoter are responsive to specific Tcf isoforms in a β-catenin-dependent manner (Atcha et al, 2003). The intriguing finding, however, that Wnt3A transcriptional induction of the Lef-1 promoter is independent of known Lef-1/Tcf-binding sites suggests that multiple Wnt-inductive mechanisms that control Lef-1 gene expression may exist (Filali et al, 2002). Given the apparent complexities of Lef-1 promoter regulation by the Wnt/β-catenin/Tcf cascade and its diverse roles in the developmental processes of multiple organ systems, in vivo studies of the Lef-1 promoter are required to more clearly dissect its regulatory components.
To elucidate regulatory elements that control Lef-1 promoter activation during development, we first evaluated transgenic mice that express a β-galactosidase reporter gene under the control of three different segments of the human Lef-1 promoter. We then analyzed the temporal and spatial patterns of β-galactosidase expression during mouse embryo development. In vivo analysis of these transgenic lines revealed that a 2.5 kb segment of the Lef-1 promoter and an internal 110 bp Wnt/β-catenin-responsive element control certain temporal and spatial developmental expression patterns of Lef-1 mRNA in hair and vibrissa follicles. Furthermore, we have shown that the 2.5 kb Lef-1 promoter segment is properly regulated during postnatal anagen phase hair-follicle cycling following induction by sonic hedgehog (Shh). Lef-1 mRNA is normally expressed in both epithelial- and mesenchymal-derived compartments of hair/vibrissa follicles. Our studies suggest that epithelial-and mesenchymal-restricted patterns of Lef-1 expression are uniquely regulated by Wnt/β-catenin-responsive sequences in the Lef-1 promoter. Cumulatively, these findings have begun to define the regulatory regions of the Lef-1 promoter that differentially control epithelial and mesench-ymal expression of the Lef-1 gene during hair/vibrissa growth.
Results
Generation of transgenic mouse lines with the human Lef-1 promoter controlling β-galactosidase reporter-gene expression
In an effort to define the minimal Lef-1-promoter sequences required for controlled developmental expression of the Lef-1 gene, three previously reported Lef-1/LacZ-reporter constructs (Filali et al, 2002) were used to generate three types of transgenic lines. The shortest construct, termed LF−2700/−200-LacZ, encompassed 2.5 kb (from −2700 to −200 bp, relative to the ATG starting codon) of the Lef-1 promoter proximal to the β-galactosi-dase gene (Fig 1B). A second longer (5.7 kb) construct (LF−2700/+3034-LacZ) encompassed −2700 to +3034bp and incorporated intron 1 and part of intron 2 from the Lef-1 gene (Fig 1A). Both of these constructs demonstrated similar promoter activity in 293 cells, including inducible expression in response to Wnt3A and β-catenin (Filali et al, 2002). The longer of the two promoter/reporter constructs, however, generated an N-terminal Lef-1/LacZ-fusion protein. A third construct (LF−2700(ID)/−200-LacZ) designed to evaluate the importance of Wnt-responsive sequences in the Lef-1 promoter was also used to generate transgenic lines (Fig 1C). These transgenic lines contained a similar reporter to LF−2700/−200-LacZ with an internal deletion between −879 and −769 bp of the Lef-1 promoter responsible for Wnt3A induction. This 110 bp element is referred to as the Wnt-responsive element (WRE). In total, five to eight independent founder lines were created from each of the three reporter constructs (Table I). All lines contained a single integration event as determined by Southern blotting of F1 progeny. The copy number of integrated transgenes, however, varied from four to 32 in different lines based on Southern blot quantification against plasmid copy number controls (Fig 1D and Table I). Two of five transgenic lines from the LF−2700/−200-LacZ construct (11434/4 and 11439/1) exhibited positive β-galacto-sidase staining during in the whisker and hair follicles of various stages of embryo development, whereas no detectable β-galactosidase, transgene expression staining was observed in the other three lines (Table I). Similarly, two of six lines derived from LF−2700/+3034-LacZ transgenic mice (11412/1 and 11416/2) also demonstrated detectable β-galactosidase transgene expression. In contrast, six of eight lines derived from the LF−2700(ID)/−200-LacZ construct (18959/3, 19351/1, 19350/1, 19298/2.2, 19304/2, and 19298/2) demonstrated detectable β-galactosidase trans-gene expression at various stages of embryogenesis. Each of the various transgenic lines were subsequently evaluated for Lef-1 promoter-LacZ developmental expression patterns in vibrissa and hair follicles (Table I) and are discussed in more detail below.
Figure 1. Generation of transgenic mice harboring human Lef-1-promoter fragments driving a LacZ reporter gene.
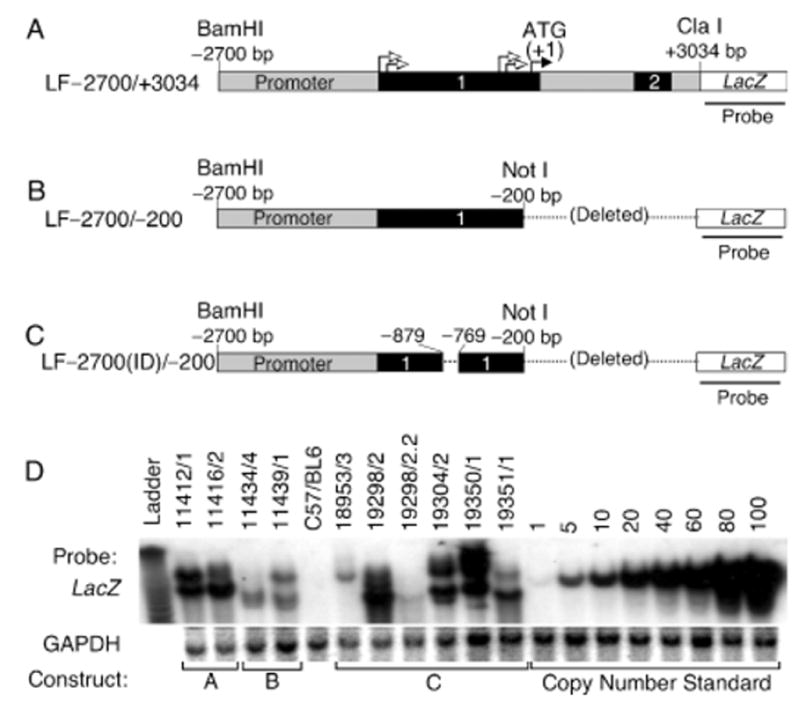
(A) Diagram of transgene fragment used to generate LF−2700/+3400 transgenic lines. This is the longest fragment of the Lef-1 gene used that contains exons 1 and 2 (shaded black), all of intron 1, and part of intron 2. The closed arrow denotes the position of the start methionine codon (+1) for the Lef-1 protein and open arrows denote the positions of four previously identified transcriptional start sites. The 3′ end of intron 2 contains a splice-acceptor site that is in frame with the LacZ transgene. (B) Diagram of the transgene fragment used to generate LF−2700/−200 transgenic lines. (C) Diagram of the transgene fragment used to generate LF−2700(ID)/−200 transgenic lines. In this construct, a 110 bp fragment encompassing a Wnt response element (−879 to −769) was deleted. (D) Southern blot analysis of all transgenic lines that expressed β-galactosidase at any time during development and which were analyzed for expression patterns at the cellular level in vibrissa and hair follicles. Genomic DNA was digested with BamHI, and Southern blots were sequentially hybridized with a LacZ and GAPDH probes. GAPDH served as an internal control for DNA loading and plasmid copy number controls were spiked into transgene-negative genomic DNA to determine transgene copy number in each line. All founders were determined to have single integration sites based on identical Southern banding patterns in all F1 and F2 generations.
Table I.
Summary of reporter gene expression in vibrissa and hair follicles
| LacZ construct | Line | Transgene copy number | Vibrissa/hair follicles (epithelial)a | Vibrissa/hair follicles (mesenchymal)b | Vibrissa/hair follicles (sebaceous glands) | Other sitesc |
|---|---|---|---|---|---|---|
| LF−2700/−200 | 11434/4 | 4 | − | + | − | + |
| LF−2700/−200 | 11439/1 | 4 | − | + | − | + |
| LF−2700/−200 | Three additional lines | ND | − | − | − | − |
| LF−2700/+ 3034 | 11412/1 | 17 | − | − | + | − |
| LF−2700/+ 3034 | 11416/2 | 21 | − | − | + | − |
| LF−2700/+ 3034 | Four additional lines | ND | − | − | − | − |
| LF−2700(ID)/−200 | 19298/2 | 4 | − | − | − | + |
| LF−2700(ID)/−200 | 18959/3 | 4 | + | − | − | + |
| LF−2700(ID)/−200 | 19351/1 | 10 | − | − | − | + |
| LF−2700(ID)/−200 | 19350/1 | 29 | − | − | − | + |
| LF−2700(ID)/−200 | 19304/2 | 32 | − | − | − | + |
| LF−2700(ID)/−200 | 19298/2.2 | 22 | − | − | − | + |
| LF−2700(ID)/−200 | Two additional lines | ND | − | − | − | − |
Expression in epithelial cells of the placode, hair germ bud, and pre-cortex.
Expression in mesenchymal cells of the mesenchymal condensate and dermal papilla.
Other sites of reporter gene expression included the brain, incisors, and limb buds.
ND, not determined.
Regulation of the 2.5 kb human Lef-1 promoter during whisker (vibrissae) morphogenesis
The arrest of hair-follicle development and the lack of whiskers are the most obvious phenotypes in Lef-1-deficient mice (van Genderen et al, 1994). Additionally, ectopic overexpression of Lef-1 in transgenic mice perturbs both whisker and hair growth, indicating that this transcription factor plays an essential role in the development of these tissues (Zhou et al, 1995). Whole-mount 5-bromo-4-chloro-3-indolyl-β-D-galactopyra-noside (X-gal) staining of transgenic embryos expressing LacZ under the 2.5 kb Lef-1 promoter (LF−2700/−200-LacZ) demonstrated β-galactosidase expression in both the whisker pad and vibrissae follicles at E12.5–15.5 of development (Fig 2A). This pattern of LacZ expression overlapped with that seen for Lef-1-mRNA expression but appeared to lag maximal peaks in mRNA expression by 1 d (Fig 2B). To better characterize expression patterns of the 2.5 kb Lef-1 promoter during whisker–follicle formation, cryosections of X-gal-stained embryos were evaluated (Fig 2C–F). Lef-1-mRNA expression during whisker and hair-follicle formation has been extensively studied and demonstrated to initiate in both epithelial and mesenchymal compartments at the earliest ectodermal–placode stage of follicular growth (Zhou et al, 1995; Kratochwil et al, 1996). Prior to whisker–follicle formation, Lef-1 protein expression is widespread throughout the whisker pad mesenchyme at E11 (Kratochwil et al, 1996). By E12, Lef-1 expression in the dermal epithelium is initiated, with concentrations at the dermal placodes where whisker–follicles will form. At E13, Lef-1 expression begins to concentrate in the mechench-yme surrounding forming dermal papilla, as well as in adjacent dermal epithelial cells. Lef-1 expression continues at the invading tips of follicles, in what appears to be a subset of both epithelial and mesenchymally derived cell types (Kratochwil et al, 1996; DasGupta and Fuchs, 1999). Lef-1 expression from E15.5 to E16.5 is then directed to mesenchymal-derived dermal papilla of the developing whisker follicle, and it finally resides primarily in the epithelial-derived matrix and pre-cortex regions of the follicular bulb (Zhou et al, 1995; Kratochwil et al, 1996; DasGupta and Fuchs, 1999).
Figure 2. Expression of the LacZ transgene in developing vibrissa follicles of the LF−2700/−200 transgenic lines.
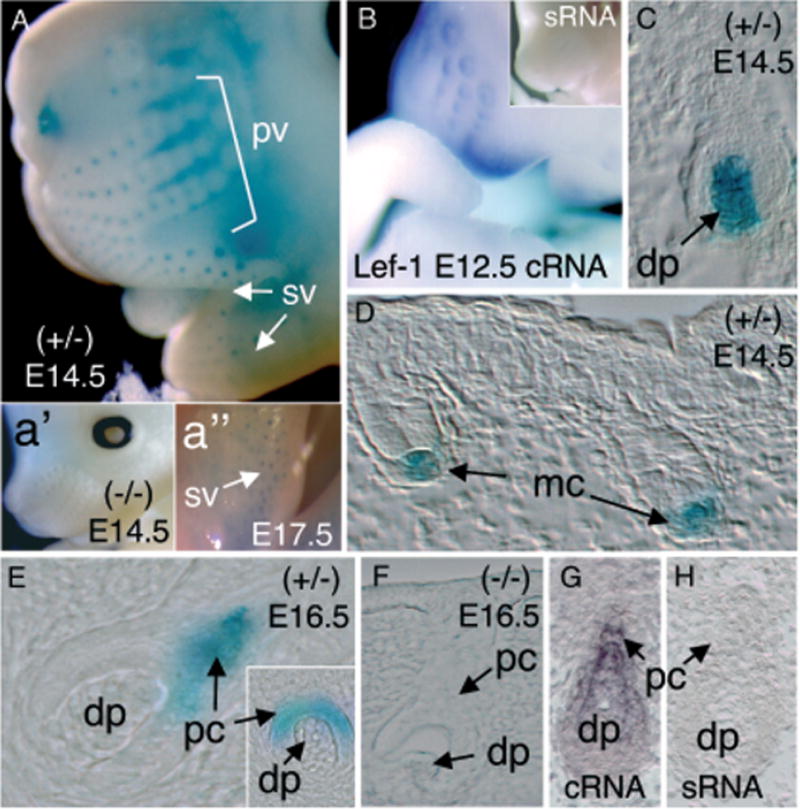
(A) Whole-mount X-gal staining of whiskers in an E14.5 transgenic embryo demonstrating positive LacZ staining in primary vibrissa follicles (pv) and supermumerary vibrissa (sv). Insets depict a lack of X-gal staining in transgene-negative littermates (a′) and persistent supermumerary-vibrissa staining in E17.5 embryos (a″). (B) Whole-mount in situ hybridization for Lef-1 mRNA using an anti-sense (cRNA) probe in an E12.5 embryo. Lef-1 mRNA was detected in vibrissa follicles and lips. No staining was observed using a sense RNA (sRNA) Lef-1 probe (inset of B). (C, D) Histologic analysis of X-gal staining in sections from E14.5 embryos demonstrating predominant Lef-1-LacZ expression in mesenchymal condensates (mc) and dermal papilla (dp) of early stage vibrissa follicles. (E) In contrast to LacZ expression observed at E14.5, Lef-1-LacZ expression was observed in the epithelial-derived pre-cortex (pc) of vibrissa in E16.5 transgenic embryo. (F) No X-gal staining was observed during vibrissa development in transgene-negative littermates at any of the time points evaluated. (G) In situ hybridization using a Lef-1 cRNA probe demonstrates Lef-1-mRNA expression in the differentiating pre-cortex (pc) and dermal papilla (dp) of vibrissa in E14.5 mouse embryos. (H) No hybridization was observed in vibrissa following hybridization of E14.5 embryos with the sense RNA (sRNA) Lef-1 probe.
Similar to these previously reported descriptions of Lef-1 mRNA expression during whisker–follicle development, transgenic mice harboring the 2.5 kb human Lef-1 promoter expressed β-galactosidase prominently in the dermal mesenchymal condensates and dermal papilla of major mystacial vibrissae at E13.5 and E14.5 (Fig 2C, D). Additionally, as expected from the endogenous Lef-1-mRNA expression patterns (Fig 2G), β-galactosidase expression from these mice decreased in the dermal papilla and increased in adjacent layers represented the differentiating pre-cortex in later stages of follicle development at E15.5 and E16.5 (Fig 2E). In contrast, no X-gal staining was observed during follicle development of transgene-negative littermates (Fig 2a′, F) or in secondary vibrissa (Rossant and Tam, 2002) (data not shown). Interestingly, the supernumerary vibrissa in the lip furrow also revealed transgene expression in mesenchymal condensates and dermal papilla at E17.5 (Fig 2a″). In contrast to the preceding similarities in Lef-1 mRNA mesenchymal expression patterns and 2.5 kb Lef-1 promoter activity, expression of the promoter was not observed in epithelial cells of the placode and hair germ bud stages (E12) where Lef-1 mRNA is normally expressed. This finding, together with the slight lag in whole-mount staining of the whiskers, suggests that the 2.5 kb Lef-1 promoter segment used may have incomplete regulatory information necessary to correctly regulate Lef-1 expression in the epithelium at the initiation stages of hair-follicle formation.
Regulation of the 2.5 kb human Lef-1 promoter during hair-follicle formation
As with vibrissa, expression of Lef-1 mRNA occurs in both mesenchymal and epithelial compartments throughout hair-follicle organogenesis (Zhou et al, 1995). Both primary hair follicles (E12–14) and secondary hair follicles (E14–17) express Lef-1. Our studies evaluating the 2.5 kb Lef-1 promoter (LF−2700/−200-LacZ) demonstrated that hair-follicle LacZ expression was only seen during the time course matching secondary hair-follicle development (E14–17). The development of the hair follicle can be classified into eight successive stages based on hair-follicle morphology and other criteria during its morphogenesis (Paus et al, 1999). At stages 1 and 2, the 2.5 kb Lef-1 promoter was expressed only within the mesench-ymal dermal condensate of hair follicles (Fig 3A, D, and I) and vibrissa (Fig 3B, E). This pattern of expression was similar to that previously observed for Lef-1 mRNA (Zhou et al, 1995; DasGupta and Fuchs, 1999). It lacked, however, epithelial-derived expression normally seen for Lef-1 protein and for mRNA in the epidermal placodes at this stage. Expression of the 2.5 kb Lef-1 promoter in stage 3 and 4 hair follicles was confined exclusively to the dermal papilla of hair and vibrissa follicles (Figs 3G, H, J, and K), a finding consistent with previous mRNA localization studies (Zhou et al, 1995). As seen in vibrissa follicles, hair follicles of the skin at greater than stage 5 no longer maintained promoter expression in the dermal papilla (data not shown), a transition also consistent with Lef-1 mRNA expression patterns (Zhou et al, 1995). Unlike vibrissa, however, the 2.5 kb promoter appeared to lack a regulated switch in expression from dermal papilla to epithelial-derived differentiating pre-cortex cells of the hair follicle in conjunction with melanin appearance (data not shown). Taken together with the lack of transgene expression in the early and late epithelial compartments of the hair follicle, our results indicate that the 2.5 kb genomic fragment of the human Lef-1 promoter does not contain all the regulatory elements necessary for appropriate epithelial expression. These results suggest that the regulation of the Lef-1 promoter in mesenchymal- and epithelial-derived compartments of the hair follicle may act through independent mechanisms that transcriptionally regulate different regions of the Lef-1 promoter.
Figure 3. Comparative expression of the Lef-1 promoter during early stage vibrissa and hair-follicle morphogenesis in the LF−2700/−200 transgenic lines.
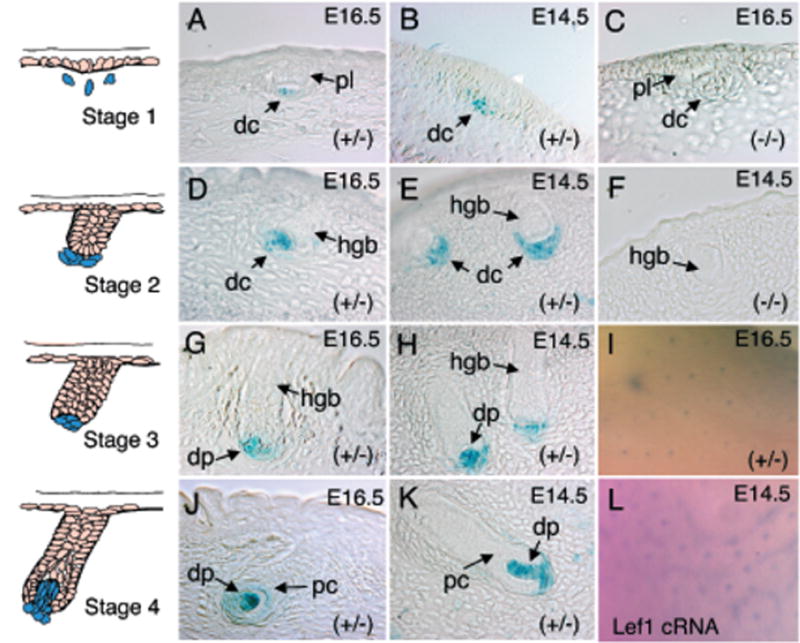
The left-hand column depicts schematic drawings of early stages of vibrissa/hair-follicle morphology (stages 1–4) (Paus et al, 1999). Photomicrographs of X-gal-stained sections are shown for each corresponding developmental stage. Representative hair follicles (A, C, D, G, and J) and vibrissa follicles (B, F, E, H, and K) from each developmental stage are shown for the given genotypes (C and F are transgene-negative controls). The embryonic stage from which each section was derived is also given in the upper right-hand corner of the figure. (I) Whole-mount X-gal staining of E16.5-transgenic mouse skin and (L) whole-mount in situ hybridization of skin using a mouse Lef-1-cRNA anti-sense probe. dc, dermal condensates; dp, dermal papilla; pc, pre-cortex; and hgb, hair germ bud; pl, placode.
Do intronic regulatory elements exist in the Lef-1 gene that controls promoter activity?
Analysis of the 2.5 kb Lef-1 promoter (LF−2700/−200) during embryogenesis has demonstrated that this region contains much, but not all, of the genetic information needed to regulate Lef-1 gene expression during hair and vibrissa development. Notable regulatory elements lacking in the 2.5 kb promoter segment (LF−2700/−200) include those responsible for initiating Lef-1 expression in the epithelium during early placode formation of the vibrissa and hair follicles. Therefore, we sought to test whether intronic sequences in the Lef-1 gene might facilitate regulated expression at these additional developmental stages of organogenesis. Six founder transgenic lines were generated from a 5.7 kb Lef-1 promoter–reporter construct (LF−2700/+ 3034-LacZ). This extended promoter fragment contained the same 2.5 kb promoter segment contained in LF−2700/−200-LacZ, in addition to intron 1 and part of intron 2. In vitro, the 2.5 and 5.7 kb promoter fragments demonstrated similar inductive responses to Wnt3A. The overall baseline level of expression for the longer promoter fragment, however, was significantly lower (Filali et al, 2002).
Of the six founder lines developed with the LF−2700/+ 3034-LacZ transgene cassette, two demonstrated selective reporter-gene expression at E17.5 and E18.5, but not at any other developmental time points. These lines displayed an identical expression pattern, as seen in whole-mount embryo staining in whisker and hair follicles (Fig 4A–D). Evaluation of cryosections demonstrated that expression was uniquely localized to sebaceous glands of the vibrissa and some hair follicles in the skin (Fig 4E–G). To identify the cell type that expressed the transgene in the sebaceous gland, we performed Oil Red O staining (Feldman and Dapson, 1974), which revealed that the positive cells were highly restricted to a subset of keratinocytes that coloca-lized with Oil Red O staining (Figs 4F, G). Both founder lines (11412/1 and 11416/2) demonstrated identical restricted-staining patterns in this region. In contrast, no X-gal staining was observed in transgene-negative control littermates (Fig 4B, D, and H).
Figure 4. Expression of the LF−2700/+ 3034 Lef-1 promoter in sebaceous glands.
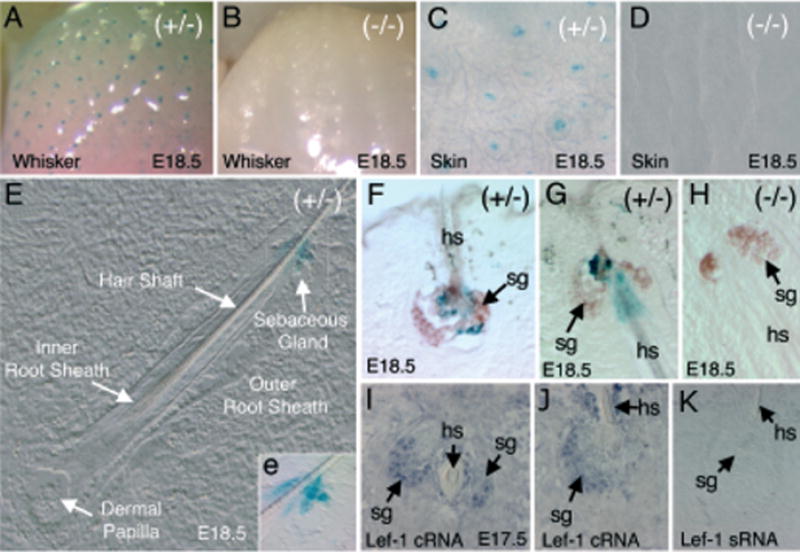
(A–D) Whole-mount X-gal staining of the whisker pad (A, B) and skin (C, D) of transgenic (A, C) and non-transgenic (B, D) embryos at E18.5. (E) Nomarski photomicrograph of a whisker cross-cryosection from a transgene-positive animal; the various structural features are annotated. The X-gal staining in the sebaceous gland is more obviously displayed in the bright field inset (e) of the enlarged boxed regions (F–H). Oil Red O-stained sections of E18.5 embryo whiskers also stained in X-gal. Sebaceous glands in whiskers stained brown using Oil Red O (arrows). (F) A cross-section and (G) sagittal-section of whiskers demonstrates a cluster of X-gal-positive cells surrounding the hair shaft in the center of the sebaceous gland from transgene-positive embryos. (H) No X-gal-positive staining was observed in whisker sebaceous glands of transgene-negative embryos. (I–K) In situ hybridization of E17.5 whiskers using anti-sense (I, J) or sense (K) mouse Lef-1-RNA probes. Lef-1 mRNA is expressed in a subset of cells within sebaceous glands (sg) surrounding the hair shaft (hs).
Lef-1 expression in sebaceous glands has not been previously evaluated. To this end, we sought to determine whether the observed pattern of Lef-1-promoter expression correlated with endogenous Lef-1-mRNA expression. Due to limited probe permeability with whole-mount in situ hybridization at these developmental time points, we performed in situ hybridization on cryosections using anti-sense (cRNA) and sense (sRNA) probes for Lef-1. Results from this study confirmed that Lef-1 mRNA was indeed expressed in sebaceous glands at E17.5 (Fig 4I, J). These data suggest that expression of the Lef-1 promoter in sebaceous glands is tightly regulated during whisker and hair-follicle development. It is currently unknown why expression of the LF−2700/+ 3034 Lef-1 promoter is turned off at other sites where expression of the shorter promoter, LF−2700/−200, is seen. These findings, however, implicate sites in the first two introns as being critical not only for sebaceous glands expression, but also in the proper regulation of upstream promoter sequences.
Wnt-responsive sequences in the Lef-1 promoter are required for mesenchymal expression during vibrissa and hair-follicle formation
Wnt3A and β-catenin responsiveness of the Lef-1 promoter in cell lines has been assigned to a 110 bp element residing at −879 to −769 bp (Filali et al, 2002). Given that numerous Wnts play a large role in hair-follicle development (Millar et al, 1999; Kishimoto et al, 2000; Huelsken et al, 2001; Reddy et al, 2001; Andl et al, 2002), we sought evaluate whether this previously characterized WRE in the Lef-1 promoter played a role in regulating transcription during vibrissa and hair morphogenesis. To this end, we performed similar developmental profiling of LacZ reporter-gene expression during vibrissa and hair-follicle development on WRE-deleted LF−2700(ID)/−200-LacZ transgenic lines (Fig 5). All transgenic lines that expressed any level of β-galactosidase during embryogenesis (E13–17) were characterized for their cellular patterns of transgene expression found during the initial stages of vibrissa and hair-follicle development (see Table I). Two of eight founders from this construct did not express the LacZ transgene in any tissue during embryo development and were not evaluated at the cellular level. Only one of the six transgene-expressing founders from the LF−2700(ID)/−200-LacZ lines (18959/3) demonstrated expression during vibrissa and hair-follicle development despite the fact the LacZ transgene was expressed in other organs such as brain and limb buds in all of these six lines during various times of embryo development (Table I).
Figure 5. Comparative expression of a WRE-deleted Lef-1 promoter (LF–2700(ID)/−200-LacZ) during early stage vibrissa and hair-follicle morphogenesis.
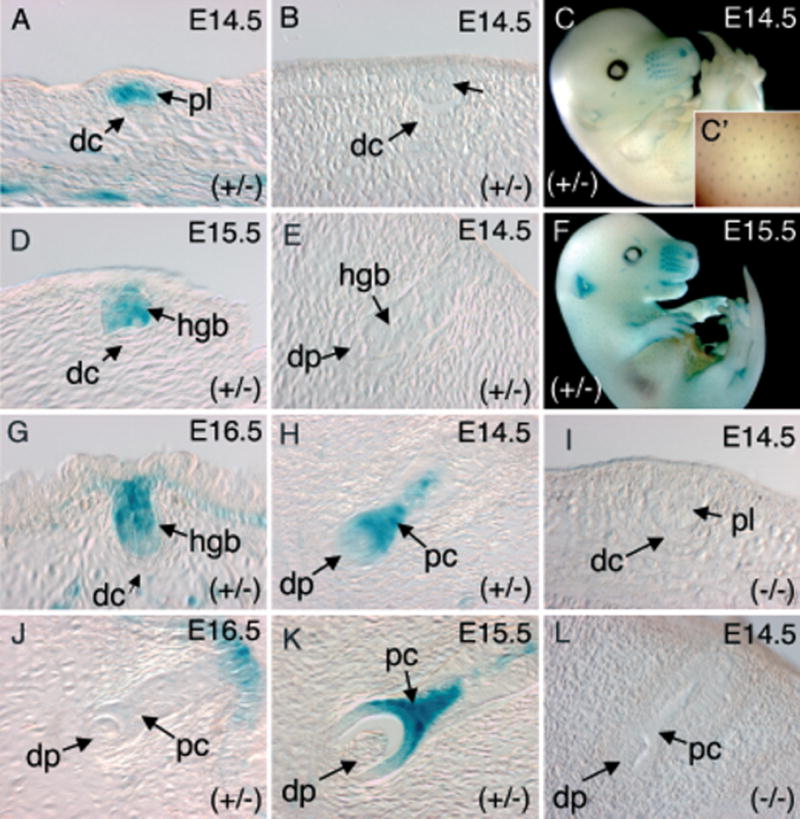
Representative X-gal staining patterns seen in transgene-positive animals for hair follicles (A, D, G, and J) and vibrissa follicles (B, E, H, and K) at different developmental stages. The embryonic stage from which each section was derived is given in the upper right-hand corner of each panel. (C, F) Whole-mount X-gal staining of E14.5 and E15.5 LF−2700(ID)/−200-LacZ transgenic embryos. (C′) Enlarged inset of X-gal-stained E14.5 skin more clearly demonstrates hair-follicle staining. (I, L) No X-gal staining was observed at any stage of hair follicles (I) and vibrissa follicles (L) of transgenic-negative embryos. dc, dermal condensates; dp, dermal papilla; pc, pre-cortex; hgb, hair germ bud; pl, placode.
The most conclusive information generated from analysis of the six transgene-expressing LF−2700(ID)/−200-LacZ lines related to the involvement of the WRE in mesenchymal expression of the Lef-1 promoter during vibrissa and hair-follicle development. In contrast to the LF−2700/−200-LacZ lines in which all of the transgene-expressing founders also expressed in the mesenchyme of developing vibrissa and hair follicles, none of the six transgene-expressing LF−2700(ID)/−200-LacZ lines gave rise to mesenchymal expression during follicle formation (Fig 5). These findings provide strong support for a requirement of the WRE in mesenchymal-restricted expression of the developing vibrissa/hair follicle. Interestingly, expression in one of the six transgene-expressing LF−2700(ID)/−200-LacZ founders (18959/3) demonstrated an epithelial-restrictive expression pattern during follicle formation that closely mirrored previously reported endogenous Lef-1 mRNA expression patterns. For example, epithelial expression during placode formation and early hair germ bud stages were clearly evident in developing hair follicles at E14.5–16.5 from this line (Fig 5A, C, D, F, and G). In contrast, mesenchymal expression was absent at these stages in the LF−2700(ID)/−200-LacZ 18959/3 line as well as the other five lines that expressed transgene at other organ sites (Table I). Although epithelial expression during the placode and hair germ stages of vibrissa development was not observed in this 18959/3 line (Fig 5B, E), epithelial expression in the pre-cortex of later stage follicles was significantly enhanced over that seen in the LF−2700/−200-LacZ lines (Fig 5H, K). These findings suggest that the WRE in the Lef-1 promoter may inhibit epithelial expression during vibrissa and hair-follicle development and perhaps sequences outside the 2.5 kb promoter are required to properly mediate this repression during development. Since epithelial expression, however, in the pre-cortex was weakly observed in a subset of vibrissa from the LF−2700/−200-LacZ lines, conclusive results determining the importance of the WRE on epithelial expression in developing follicles would require that a greater number of transgene-expressing founders be analyzed. It is also possible that random differences in transgene integration sites, and their surrounding chromatin, between the various constructs and founders analyzed may influence WRE function in our Lef-1 promoter–reporter constructs.
Several conclusions and hypotheses from these studies evaluating the importance of the WRE in vibrissa/hair-follicle expression from the Lef-1 promoter can be drawn. The lack of mesenchymal expression in dermal condensates and dermal papilla from all the LF−2700(ID)/−200-LacZ lines demonstrates that the WRE in the Lef-1 promoter is required for expression in these cell types. Given the previous identified function of the WRE in vitro (Filali et al, 2002), these finding suggest that Wnt signals may mediate activation of the Lef-1 promoter in the mesenchyme of developing vibrissa and hair follicles. Second, it is clear that sequences outside the 2.5 kb Lef-1 promoter are necessary for proper regulation of epithelial expression during follicular development. Lastly, the 110 bp WRE in the Lef-1 promoter may play a role in mediating transcriptional switching between mesenchymal and epithelial compartment during follicle morphogenesis. Further work, however, is required to support this hypothesis conclusively.
Expression from the 2.5 kb Lef-1 promoter segment is induced during the anagen stage of hair-follicle cycling
Hair follicles cycle through three major stages, including a growth stage (anagen), an apoptosis-driven regression stage (catagen), and a rest stage (telogen). Numerous signaling molecules, including Shh, Wnt, β-catenin, Lef-1, FGFs, BMPs, noggin, and versican, have been identified as playing important roles in both hair-follicle morphogenesis and cycling (Botchkarev et al, 1999; Kishimoto et al, 1999; Paus and Cotsarelis, 1999; Kishimoto et al, 2000; Muller-Rover et al, 2001). Shh has also been demonstrated to play a critical role in inducing a switch from telogen to anagen stage of the hair follicle. Ectopic expression of Shh from a recombinant adenoviral vector has been shown to induce anagen-stage growth of telogen-synchronized hair follicles of 18 d postnatal mice. (Dlugosz 1999; Sato et al, 1999, 2001). Results demonstrating that the 2.5 kb human Lef-1 promoter regulates transgene expression only at early stages of follicle morphogenesis prompted us to investigate whether this segment of the promoter was also properly regulated during postnatal hair-follicle cycling. The link between Shh and Lef-1 induction during hair-follicle morphogensis is also supported by the similar phenotypes observed in null mice for each of these genes (van Genderen et al, 1994; Sato et al, 1999). Furthermore, although the 2.5 kb Lef-1 promoter lacked epithelial-specific regulation during the early placode stage of hair-follicle development, its pattern of expression closely mirrored the phases in which Shh plays a critical role in follicle morphogenesis. Hence, we hypothesized that the 2.5 kb Lef-1 promoter segment might contain regulatory elements responsive to downstream signals from Shh.
To test the hypothesis that the 2.5 kb Lef-1 promoter (LF−2700/−200-LacZ) was properly regulated during ana-gen-stage growth initiated by Shh signals, we examined whether ectopic expression of Shh in the skin could induce the Lef-1 promoter concordant with the induction of hair-follicle growth. Recombinant adenovirus was used as the delivery vehicle for Shh. Preliminary studies using an Ad.LacZ-control vector demonstrated significant LacZ expression in the dermis and panniculus carnosus muscle following intradermal delivery of the adenoviral vector (Fig 6A, B). Furthermore, intradermal injection of Ad.Shh significantly induced hair growth in postnatal day 18 mice, as compared to mice injected with the control empty adenoviral vector (Ad.BglII) (Fig 6C, D). These results confirm previous reports demonstrating the ability of ectopically expressed Shh to promote hair cycling to anagen stage around the injection site (Sato et al, 1999).
Figure 6. Shh induces anagen-stage hair-follicle growth and expression of the LF−2700/−200 transgenic Lef-1 promoter in dermal papilla.
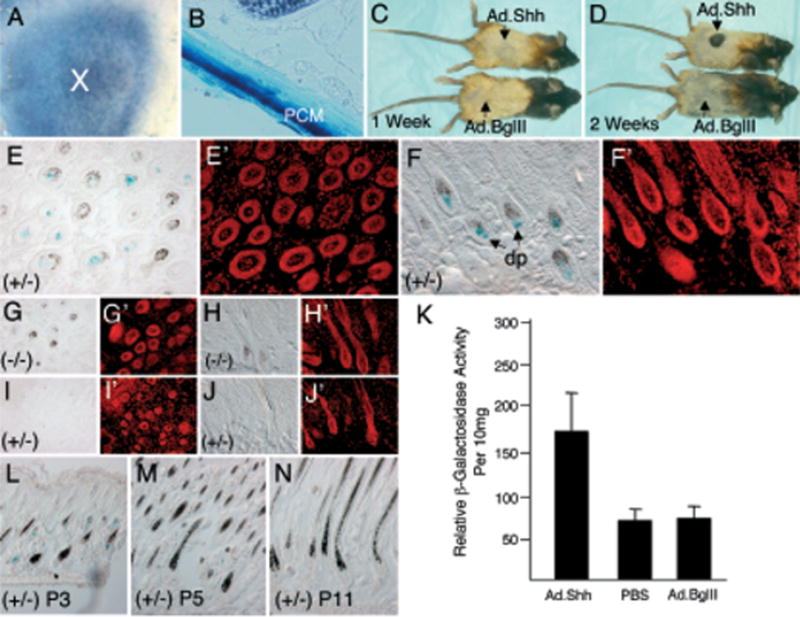
Intradermal administration of Ad.LacZ gave rise to significant β-galactosidase activity at 5 d post-infection in (A) X-gal-stained whole-mount skin (X-marks injection site) and (B) sagittal section of the skin. LacZ expression was predominant in the panniculus carnosus muscle (PCM) of skin. (C, D) Hair growth in C57BL/6 mice after intradermal administration of Ad.Shh during the first telogen stage of hair cycling. Dorsal skins of day 18 postnatal C57BL/6 were injected (arrows) with Ad.Shh (top) or Ad.BglII-null virus (bottom). The dorsal hair was bleached to provide light color contrast for assessing the new growth of black hair (Sato et al, 1999). Melanogenesis was observed at the injection site of Ad.Shh, but not in Ad.BglII-injected mice at 1 wk post-infection (C). New hair growth (black area) became visible at sites of Ad.Shh, but not Ad.BglII, injection sites 2 wk after the administration of virus (D). Morphologic evaluation of X-gal staining patterns in Ad.Shh-infected transgenic mice (E, F), Ad.Shh-infected non-transgenic mice (G, H), and Ad.BglI-infected transgenic mice (I, J). All were X-gal stained at 5 d post-intradermal administration of the adenovirus. (E′, F′, G′, H′, I′; and J′) Propidium iodide-stained fluorescent image of the corresponding X-gal-stained panel on the right. (K) Quantification of β-galactosidase activity in dermal-cell lysates at 5 d post-injection with PBS, Ad.BglII or Ad.Shh virus. Results depict the mean (± SEM) for four samples in each group. (L, M, and N) Expression of the Lef-1 promoter in skin on postnatal day 3 (L), day 5 (M), and day 11 (N) in the absence of ectopic Shh expression. The Lef-1 promoter is abundantly expressed in dermal papilla of anagen stage hair follicles on postnatal day 3. dp, dermal papilla; (±), heterozygous for the Lef-1-LacZ transgene; (−/−), transgene negative.
Consistent with the original hypothesis, cryosections of Ad.Shh-infected skin demonstrated induced β-galactosi-dase expression in the dermal papillae of anagen-stage follicles (Fig 6E, F). Serial section demonstrated that all hair follicles in anagen-stage-induced LacZ expression from the Lef-1 promoter, suggesting that the induction was stage specific. No X-gal-positive staining was seen in anagen hair follicles of Ad.Shh-infected, non-transgenic mice (Fig 6G, H) or in telogen-stage hair follicles of Ad.BglII-infected trans-genic mice (Fig 6I, J). These findings of anagen-stage induction of the 2.5 kb Lef-1 promoter were further supported by quantitative β-galactosidase enzymatic assays and demonstrated a 2-fold induction of transgene expression following Shh expression (Fig 6K). In summary, these results demonstrate that Shh is capable of indirectly inducing expression of the 2.5 kb human Lef-1 promoter during hair-follicle cycling. It should be noted, however, that expression of the Lef-1 promoter in dermal papilla is a feature of anagen-stage follicle cycling and not directly an affect of Shh action on the promoter. In this context, Shh expression synchronizes follicles in anagen stage and hence increases the abundance of follicles that express the Lef-1 promoter. In support of this notion, anagen-stage hair follicles are also in high abundance at postnatal day 3 and these follicles also express LacZ in the dermal papilla without exogenous Shh (Fig 6L). As hair follicles progress to telogen stage at days 5–11 postnatally (Fig 6M, N), expression of the Lef-1 promoter is progressively turned off.
Discussion
Lef-1 mRNA and protein expression are highly regulated during the development of many organ systems. Recent in vitro studies have demonstrated that the Lef-1 promoter is transcriptionally responsive to Wnt/Tcf/β-catenin induction (Filali et al, 2002; Atcha et al, 2003). Although it is generally accepted that transcriptional regulation of the Lef-1 gene plays an important role in vivo during development of many organs, it is also possible that post-transcriptional and/or post-translational mechanisms also regulate Lef-1-mRNA or protein levels, respectively. To begin to dissect the Lef-1 developmental pathways that are regulated at the level of Lef-1-gene transcription, we have initiated in vivo analyses of three Lef-1 promoter-LacZ reporter constructs in the skin of transgenic mice.
Analysis of the shorter 2.5 kb human Lef-1 promoter construct (LF−2700/−200-LacZ) during mouse embryogenesis suggests that this region contains only some of the genetic information needed to regulate Lef-1 transcription during whisker and hair-follicle development. Expression patterns from this construct were consistent between the two lines evaluated. In contrast, the longer 5.7 kb Lef-1 promoter construct (LF−2700/+ 3034-LacZ), which also contained some intronic sequences, demonstrated a very restricted pattern of expression in the sebaceous gland. One explanation for this finding may be that inhibitory regulatory elements exist within the first two introns that limit expression from the promoter when they are taken out of the context of the full-length gene. The reduced expression of this larger promoter fragment is also consistent with cell-line studies (Filali et al, 2002). A second possibility is that this construct creates a Lef-1/LacZ-fusion protein product that might interfere with Lef-1-promoter regulation. The N-terminal sequences of Lef-1 in exons 1 and 2 that fuse to LacZ contain portions of the β-catenin-binding domain. Since β-catenin and Tcf have been implicated in Lef-1-promoter regulation in cell line studies, this could conceivably interfere with Tcf signaling at the Lef-1 promoter. Since no overt abnormalities, however, were seen in the development of embryos from this LF−2700/+ 3034-LacZ line, such a dominant phenotype, if it exists, must be very subtle.
Although our data indicate that the 2.5 kb Lef-1 promoter fragment does not completely mimic the temporal and spatial expression of the endogenous Lef-1 gene during morphogenesis of whisker and hair follicles, several consistent sites of expression from this promoter fragment correlated with endogenous Lef-1-mRNA expression patterns. The full-length 2.5 kb Lef-1-promoter fragment demonstrated a restricted pattern of expression in me-senchymal-derived cells of dermal condensates and dermal papillae during the formation of vibrissa and hair follicles. In contrast, analysis of the WRE-deleted 2.5 kb Lef-1-promoter construct (LF−2700(ID)/−200-LacZ) demonstrated a consistent lack of expression in mesenchymal-derived cells of dermal condensates and dermal papillae in all founders. This finding suggests that Wnt signaling may act to induce the Lef-1 promoter in mesenchymal cells of developing follicles by acting on the WRE. Although it is clear the WRE in the Lef-1 promoter is required for expression in mesenchymal components of developing follicles, the influence of the WRE on epithelial expression remains a somewhat open question. Our data demonstrating a single founder from the LF−2700(ID)/−200-LacZ line with a restricted pattern of expression in epithelial-derived placodes, hair germ buds, and pre-cortex during the formation of vibrissa and hair follicles, suggest the WRE may be a repressor of epithelial specific expression although simultaneously is required for mesenchymal induction during follicle morphogenesis. In support of this hypothesis, in vitro studies evaluating transcriptional regulation of the Lef-1 promoter in epithelial cells have also found that the WRE functions as both a repressor of baseline transcription and inductive element of Wnt-signaling (Filali et al, 2002). Furthermore, our analysis of 19 founders evaluated in this study from three Lef-1 promoter/reporter constructs, supports the notion that the WRE generally reduced expression of the Lef-1 promoter at any site in the embryo (six of eight or 75% of WRE-deleted founders expressed transgene in embryos where four of 11 or 36% of WRE-inclusive founders expressed transgene in embryos) (Table I). Cumulatively, these studies begin to shed light on the complexities of Lef-1-promoter regulation and suggest the promoter is likely regulated by independent transcriptional modules in epithelial and mesenchymal cell types. The coordinated regulation of this promoter in these two cellular compartments may play an important role in known functions of Lef-1 to mediate epithelial–mesenchymal interactions during development.
One of the most notable sites of expression from LF−2700/−200 promoter fragment was within the dermal papilla of newly forming whisker and hair follicles. This region is composed of specialized mesenchymal fibro-blasts, located at the base of follicles, that govern cell fate and differentiation during hair-follicle organogenesis and cycling (Zhou et al, 1995; Paus and Cotsarelis, 1999). Epithelial-derived signaling molecules, such as FGFs, BMPs, and Shh, invoke signaling cascades and mesench-ymal-derived factors, such as versican, noggin, and Alx4, within the dermal papilla. Striking similarities between the expression of known dermal papilla-signaling intermediates (Alx4, versican, and noggin) and the expression of the 2.5 kb Lef-1 promoter were observed in our study. This region of the Lef-1 promoter was highly expressed in condensing mesenchymal cells adjacent to the invaginating hair germ epithelium of newly forming follicles in a fashion similar to Alx4 (Boras and Hamel, 2002), an identified co-factor that binds Lef-1. Similarly, expression of the 2.5 kb Lef-1 promoter was observed at sites of reported versican expression. The versican promoter also exhibits dermal papilla-specific expression in hair follicles of both embryonic and postnatal skin (Kishimoto et al, 1999). Noggin, an inhibitor of BMP action, is a mesenchymal-derived stimulator of hair-follicle induction, for which expression is also strikingly restricted to the dermal papilla of developing hair follicles. Recent findings demonstrating that expression of Lef-1 expression is reduced in noggin-null mice strongly suggest that Lef-1 expression might be repressed by BMP (Botchkarev et al, 2002). Other studies also support the modulation of BMP and Wnt/β-catanin/Lef-1-signaling pathways in the control of hair-follicle morphogenesis (van Genderen et al, 1994; Botchkarev et al, 1999, 2002). Hence, our results are consistent with the presently known transcriptional networks that control gene expression in the dermal papilla and suggest that similar transcriptional networks may regulate the Lef-1 and versican promoters.
Mice deficient in Shh, Lef-1, or noggin exhibit a similar phenotype during hair-follicle morphogenesis: development of the follicle is retarded at an early stage, just prior to hair-shaft formation (van Genderen et al, 1994; St-Jacques et al, 1998; Botchkarev et al, 1999). Evidence suggests that Shh is not required for the initial stages of hair–placode formation and in growth from the epidermis. Shh, however, is essential for hair-follicle morphogenesis and postnatal cycling (St-Jacques et al, 1998; Sato et al, 1999; Kenney and Rowitch, 2000). Although Shh, certain Wnts, and Lef-1 are spatially regulated in a similar fashion during hair-follicle development, it is presently unclear whether the induction of Shh lies proximal or distal to Wnt induction of the Lef-1/β-catenin pathways. Our data demonstrated that ectopic expression of Shh can induce anagen-stage cycling and the appropriate induction of the 2.5 kb Lef-1 promoter in dermal papillae. This result is somewhat consistent with the findings that Wnt/β-catenin pathways induce the 2.5 kb Lef-1 promoter in cell lines (Filali et al, 2002) and that Shh-knockout mice lack Wnt5a-mRNA induction during hair-follicle morphogenesis (Reddy et al, 2001). We have found, however, that ectopic expression of Wnt3A with recombinant adenovirus failed to induce Lef-1 promoter expression or hair growth in 18 d postnatal skin (data not shown). Other reports appear to place Shh induction downstream of Wnt/β-catenin/Lef-1 induction since Shh expression is absent in hair follicles of β-catenin-targeted deletion mice (Huelsken et al, 2001). Regardless of the mechanisms of Shh action, our data clearly demonstrates that the 2.5 kb Lef-1 promoter is properly regulated during anagen-stage hair-follicle cycling. Despite the identified importance of the WRE for epithelial and mesenchymal expression during hair-follicle development, these sequences do not appear to be responsive to Wnt3A in vivo during post-natal hair cycling. It is, therefore, likely that other Wnts play a more predominant role at these stages in regulating Lef-1 promoter transcription at the WRE.
Epithelial–mesenchymal interactions play an important role in the development of many organs formed from epithelial-derived buds. In this context, Lef-1 and numerous other regulatory factors are required for reciprocal interactions between the epithelium and the underlying mesenchyme. These factors are important in initiating bud formation, tubule elongation, and cellular differentiation. It has long been assumed that transcriptional regulation of the Lef-1 gene plays a crucial role in each of these processes. Studies that directly evaluate the regulatory properties of the Lef-1 promoter in vivo, however, have been lacking. The present study has begun to define the cellular patterning of Lef-1 gene regulation, which can now be directly assigned to transcriptional regulation of the Lef-1 promoter. Our data suggests that sequences present between −2700 to −200 bp of the Lef-1 promoter control both epithelial and mesenchymal expression during vibrissa and hair-follicle development. The WRE in this promoter segment, however, appears to influence both epithelial and mesenchymal expression through distinct regulatory pathways. Additional sequences outside (upstream or downstream) the 2.5 kb promoter segment evaluated in our study are likely necessary for proper transcriptional regulation of the WRE. Studies demonstrating Lef-1 promoter transcription is also influenced by intronic sequences that promote expression in sebaceous glands, while simultaneously inhibiting expression at other sites in vibrissa and hair follicles, support the notion that regulatory elements which coordinate expression of the Lef-1 promoter likely interact from distant sites in the gene. In conclusion, our results demonstrate that Wnt/β-catenin-responsive sequences in the Lef-1 promoter play an important role in coordinating epithelial and mesenchymal expression during vibrissa/hair-follicle development through unique transcriptional pathways.
Materials and Methods
Lef-1 promoter–reporter constructs and the generation of trans-genic mice
Three previously described expression constructs (LF−2700/−200, LF−2700/+ 3034, and LF−2700(ID)/−200-LacZ) containing the human Lef-1 promoter fragments upstream to the β-galactosidase gene were used to generate transgenic mice (Filali et al, 2002). LF−2700/−200 contains sequences in the promoter that span −2700 to −200 bp (relative to the ATG start codon at + 1). LF−2700/+ 3034 contains the 5′ Lef-1 promoter sequence to −2700 bp, along with additional sequences of the Lef-1 gene to intron 2 (+ 3034 bp). LF−2700/+ 3034 generates an in-frame spliced mRNA product between exons 1 and 2 of the Lef-1 gene and a synthetic acceptor splice site generated 5′ to the LacZ cDNA. LF−2700(ID)/−200-LacZ has an internal 110 bp deletion of a previously characterized Wnt/β-catenin-responsive element (Filali et al, 2002). Transgenic fragments, lacking all plasmid sequences, were excised from the parental plasmid and gel purified using the Qiagen Gel Extraction kit (Qiagen, Valencia, California). Transgenic founders were generated by injecting the linear transgene cassette into the pronucleus of fertilized F2 oocytes from (C57BL/6J X SJL/J) F1 parents (Jackson Lab, Bar Harbor, Maine). Embryos were subsequently transferred into ICR pseudopregnant females (Harlen, Indianapolis, Indiana). Transgenic founders and progeny were screened by PCR and/or Southern blotting using DNA prepared from tail biopsies. Primer sets EL905 5′CAAACTTCAGCTTCCCTTCTGCTG and EL906 5′GACGAGGAAGAAGGAACTGAAGAC were derived from a β-galactosidase transgene and used for PCR screening. These primers identified a 379 bp β-galactosidase transgene band in positive transgenic mice. The transgene copy number was determined by Southern blot hybridization intensity of mouse genomic tail DNA against a β-galactosidase probe and compared to hybridization signals obtained with defined plasmid copy number controls (spiked into transgene-negative tail DNA) using a phosphoimager. A mouse GAPDH DNA fragment was used as a probe to verify equal DNA loading between founders. All animal experiments were monitored regularly and maintained in accordance with the principles and procedures outlined in the NIH guidelines for the care and use of experimental animals.
Histochemical detection of β-galactosidase activity
Timed pregnancies were established between C57BL/6J males and heterozygous, transgene-positive females. The appearance of a vaginal plug in the morning following breeding was designated as E0.5 d. The genotype of the embryos was determined by PCR of DNA harvested from the yolk sac or part of the embryo. Embryos and/or tissue samples were dissected in cold phosphate-buffered saline (PBS) at designated ages and fixed (0.2% glutaraldehyde, 5 mM EGTA pH 7.3, 2 mM MgCl2, 0.02% NP40, 0.01% deoxycholate, 2% paraformaldehyde 0.1 M sodium phosphate pH 8.8) for 15–60 min at room temperature with rocking. After being washed in detergent solution (0.02% NP40, 0.01% deoxycholate, 2 mM MgCl2, in 0.1 M sodium phosphate pH 8.0) three times for 10 min at room temperature, the embryos were stained in X-gal solution (1 mg per mL X-gal, 5 mM potassium ferricyanide, and 5 mM potassium ferrocyanide in the wash buffer) in the dark at 37°C for 3 h. They were then incubated at room temperature overnight. The stained embryos were post-fixed in 0.5% glutar-aldehyde/10% formalin after being washed three times in PBS. Embryos were equilibrated in 80% glycerol/PBT (PBS containing 0.5% Triton X-100) prior to being photographed. When histologic sections were evaluated, the embryos were equilibrated in 30% sucrose and embedded in optimal temperature cutting (OTC) compound. For X-gal staining of cryosection (12 μm), sections were fixed in 2% paraformaldehyde for 1–2 min, washed five times for 5 min, and X-gal stained at 37°C overnight, as described above. Sections were counterstained with propidium iodide, Oil Red O, or H&E prior to being photographed.
In situ hybridization
The proline-rich region (450 bp) of the mouse Lef-1 cDNA (Genebank GI:6754531) was cloned into a pBlueScript KS plasmid (Stratagene, La Jolla, California) using KpnI and EcoR V-restriction sites. This region does not overlap with conserved HMG DNA-binding domains. Mouse Lef-1 sense and anti-sense, Digoxigenin-labeled RNA probes were prepared by in vitro transcription using a Dig-RNA labeling kit with T3 and T7 RNA polymerase per manufacturer’s instructions, respectively (Roche Molecular Biochemicals, Mannheim, Germany). Whole-mount hybridization was performed essentially as previously described (Hogan 1994; Wassarman and Depamphilis 1993), however, a higher temperature (70°C) was employed during hybridization and washing steps. The proteinase-K incubation times (5–60 min) were also adjusted for different stages of embryos. Embryos were photographed after dehydration in a series of 25%, 50%, 75%, and 100% methanol, after which they were cleared in 80% glycerol. In situ hybridizations of skin and whiskers were performed on cryosection as previously described (Engelhardt et al, 1995; Duan et al, 1998; Duan et al, 1999) with Digoxigenin-labeled RNA probes.
Shh induction of anagen-stage hair-follicle growth
A recombinant adenoviral vector that expresses Sonic hedgehog (Ad.Shh) was kindly provided by Dr Ronald G. Crystal at Cornell University (Sato et al, 1999; Bergstein et al, 2002). Control adenoviral vectors included Ad.BglII (empty vector) and Ad.LacZ, which expressed β-galactosidase (Zhou, et al, 2001). Recombinant adenoviral vectors were amplified in 293 cells, purified by two rounds of CsCl banding, and titered as described elsewhere (Dracopoli 2001). To evaluate the inducibility of our human Lef-1 promoter in vivo during anagen-stage hair-follicle growth, we performed intradermal injections of 108 PFU per 20 μL of Ad.Shh, Ad.BglII, or Ad.CMV-LacZ into the dorsal skin of 18 d, postnatal transgenic animals (Sato et al, 1999). Skin-tissue samples were harvested at 5 d post-infection, X-gal stained, and embedded for frozen sectioning. For enzymatic quantification of β-galactosidase activity, a 1 cm diameter region of skin from the injected site was homogenized in lysis solution (100 mM potassium phosphate, 0.2% Triton X-100, 0.3 mM DTT, 0.2 mM PMSF, pH 7.8), and the extracts (supernatant) were heated at 48°C for 1 h to inactivate endogenous β-galactosidase activity. β-galactosidase assays were then performed using the Galacto-Light System (Applied Biosystems, Bedford, Massachussets) essentially as described by the manufacturer’s protocol.
Abbreviations
- LEF-1
Lymphoid Enhancer Factor 1
- Shh
sonic hedgehog
- WRE
Wnt-responsive element
- X-gal
5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside
Footnotes
We would like to thank Ronald Crystal and Phil Leopold for the use of the Shh adenoviral construct. We also greatly appreciate the technical assistance of Trish Lovell, Norma Sinclair, and Jessica Humberd in the Transgenic Core Facility at University of Iowa and the editorial assistance of Leah Williams. This work was funded by an NIDDK grant, DK47967 (J.F.E.), and a Gene Therapy Core Center grant through the Transgenic Core Facility, NIDDK grant DK54759.
References
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Atcha FA, Munguia JE, Li TW, Hovanes K, Waterman ML. A new beta-catenin-dependent activation domain in T cell factor. J Biol Chem. 2003;278:16169–16175. doi: 10.1074/jbc.M213218200. [DOI] [PubMed] [Google Scholar]
- Bergstein I, Leopold P, Sato N, Panteleyev A, Christiano A, Crystal R. In vivo enhanced expression of patched dampens the sonic hedgehog pathway. Mol Ther. 2002;6:258–264. doi: 10.1006/mthe.2002.0628. [DOI] [PubMed] [Google Scholar]
- Billin AN, Thirlwell H, Ayer DE. Beta-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol Cell Biol. 2000;20:6882–6890. doi: 10.1128/mcb.20.18.6882-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boras K, Hamel PA. Alx4 binding to LEF-1 regulates N-CAM promoter activity. J Biol Chem. 2002;277:1120–1127. doi: 10.1074/jbc.M109912200. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, et al. Nat Cell Biol. 1:158–164. doi: 10.1038/11078. java/Propub/cellbio/ncb0799_158.fulltext java/Propub/cellbio/ncb0799_158. abstract, 1999. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Sharov AA, Funa K, Huber O, Gilchrest BA. Modulation of BMP signaling by noggin is required for induction of the secondary (nontylotrich) hair follicles. J Invest Dermatol. 2002;118:3–10. doi: 10.1046/j.1523-1747.2002.01645.x. [DOI] [PubMed] [Google Scholar]
- Brantjes H, Roose J, van De Wetering M, Clevers H. All Tcf HMG box transcription factors interact with Groucho-related co- repressors. Nucleic Acids Res. 2001;29:1410–1419. doi: 10.1093/nar/29.7.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Dlugosz A. The hedgehog and the hair follicle: A growing relationship. J Clin Invest. 1999;104:851–853. doi: 10.1172/JCI8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracopoli NC. Current Protocols in Human Genetics. New York: Wiley; 2001. [Google Scholar]
- Duan D, Sehgal A, Yao J, Engelhardt JF. Lef1 transcription factor expression defines airway progenitor cell targets for in utero gene therapy of submucosal gland in cystic fibrosis. Am J Respir Cell Mol Biol. 1998;18:750–758. doi: 10.1165/ajrcmb.18.6.2987. [DOI] [PubMed] [Google Scholar]
- Duan D, Yue Y, Zhou W, Labed B, Ritchie TC, Grosschedl R, Engelhardt JF. Submucosal gland development in the airway is controlled by lymphoid enhancer binding factor 1 (LEF1) Development. 1999;126:4441–4453. doi: 10.1242/dev.126.20.4441. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]
- Feldman AT, Dapson RW. Relative effectiveness of various solvents for oil red O. Med Lab Technol. 1974;31:335–341. [PubMed] [Google Scholar]
- Filali M, Cheng N, Abbott D, Leontiev V, Engelhardt JF. Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J Biol Chem. 2002;277:33398–33410. doi: 10.1074/jbc.M107977200. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Merrill BJ, Jamora C, DasGupta R. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Hogan B. Manipulating the Mouse Embryo: A Laboratory Manual. 2. Plainview: Cold Spring Harbor Laboratory Press; 1994. pp. xvii–497. [Google Scholar]
- Hsu SC, Galceran J, Grosschedl R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with beta-catenin. Mol Cell Biol. 1998;18:4807–4818. doi: 10.1128/mcb.18.8.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. Beta-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol. 2000;20:9055–9067. doi: 10.1128/mcb.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE. Selective activation of the versican promoter by epithelial–mesenchymal interactions during hair follicle development. Proc Natl Acad Sci USA. 1999;96:7336–7341. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, Barsh GS. WNT signaling in the control of hair growth and structure. Dev Biol. 1999;207:133–149. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Novak A, Hsu SC, Leung-Hagesteijn C, et al. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways. Proc Natl Acad Sci USA. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterwegel M, van de Wetering M, Clevers H. HMG box proteins in early T-cell differentiation. Thymus. 1993;22:67–81. [PubMed] [Google Scholar]
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Paus R, Muller-Rover S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PPL. Mouse Development: Patterning, Morphogenesis, and Organogenesis. San Diego: Academic Press; 2002. pp. xviii–712. [Google Scholar]
- Sato N, Leopold PL, Crystal RG. Induction of the hair growth phase in postnatal mice by localized transient expression of sonic hedgehog. J Clin Invest. 1999;104:855–864. doi: 10.1172/JCI7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Leopold PL, Crystal RG. Effect of adenovirus-mediated expression of Sonic hedgehog gene on hair regrowth in mice with chemotherapy-induced alopecia. J Natl Cancer Inst. 2001;93:1858–1864. doi: 10.1093/jnci/93.24.1858. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, DePamphilis ML. Guide to techniques in mouse development. In: Wassarman PM, DePamphilis ML, editors. Methods in Enzymology. Vol. 225. San Diego: Academic Press; 1993. pp. xxxviii–373. [Google Scholar]
- Yasumoto K, Takeda K, Saito H, Watanabe K, Takahashi K, Shibahara S. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. 2002;21:2703–2714. doi: 10.1093/emboj/21.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Byrne C, Jacobs J, Fuchs E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 1995;9:700–7013. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM, Engelhardt JF. Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology. 2001;33:902–1914. doi: 10.1053/jhep.2001.23073. [DOI] [PubMed] [Google Scholar]


