Abstract
T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) was inhibitory to four strains of avian H5N1 influenza virus in MDCK cells, with the 90% effective concentrations ranging from 1.3 to 7.7 μM, as determined by a virus yield reduction assay. The efficacy was less than that exerted by oseltamivir carboxylate or zanamivir but was greater than that exerted by ribavirin. Experiments with mice lethally infected with influenza A/Duck/MN/1525/81 (H5N1) virus showed that T-705 administered per os once, twice, or four times daily for 5 days beginning 1 h after virus exposure was highly inhibitory to the infection. Dosages from 30 to 300 mg/kg of body weight/day were well tolerated; each prevented death, lessened the decline of arterial oxygen saturation (SaO2), and inhibited lung consolidation and lung virus titers. Dosages from 30 to 300 mg/kg/day administered once or twice daily also significantly prevented the death of the mice. Oseltamivir (20 mg/kg/day), administered per os twice daily for 5 days, was tested in parallel in two experiments; it was only weakly effective against the infection. The four-times-daily T-705 treatments at 300 mg/kg/day could be delayed until 96 h after virus exposure and still significantly inhibit the infection. Single T-705 treatments administered up to 60 h after virus exposure also prevented death and the decline of SaO2. Characterization of the pathogenesis of the duck influenza H5N1 virus used in these studies was undertaken; although the virus was highly pathogenic to mice, it was less neurotropic than has been described for clinical isolates of the H5N1 virus. These data indicate that T-705 may be useful for the treatment of avian influenza virus infections.
The recently reported outbreaks of highly pathogenic avian influenza occurring in Southeast Asia and the ability of the influenza viruses to transfer through bird populations and to humans have provoked much concern that such infections could lead to another influenza pandemic (8, 9, 26). Four drugs have been approved for use for the treatment of influenza virus infections in the clinic; these are the M2 ion channel inhibitors amantadine and rimantadine and the influenza virus neuraminidase (NA) inhibitors oseltamivir and zanamivir. Recent studies have indicated, however, that many of the avian influenza H5N1 viruses are resistant to both amantadine and rimantadine (25), with the viruses having an M gene containing mutations associated with this resistance (14). Researchers have now reported that zanamivir is significantly less effective against experimental infections induced in mice with the highly pathogenic avian influenza virus (10), requiring 10-fold or higher dosages to render the same protective effect observed with other influenza viruses, and oseltamivir's efficacy is also significantly less when it is used to treat mice infected with the more virulent H5N1 virus (27). The potential neurotropism of the viruses (6) raises further concerns regarding the capability of the current NA inhibitors to be efficacious, since their ability to cross the blood-brain barrier is in question (22). Finally, it is anticipated that the development of some viruses resistant to oseltamivir and zanamivir will occur, based on past experiences with the use of these drugs against more traditional influenza virus infections (7). Thus, a need continues to exist for additional antiviral agents with the ability to inhibit infections induced by the avian H5N1 viruses.
The substituted pyrazine 6-fluoro-3-hydroxy-2-pyrazinecarboxamide (T-705) has been reported by Furuta et al. (4) and by Takahashi et al. (23) to have potent and selective inhibitory activity against influenza A (H1N1, H2N2, H3N2), B, and C viruses in vitro and to significantly inhibit an infection in mice induced by influenza A/PR/8/34 (H1N1) virus in mice. Later studies indicated that the primary mechanism of action of T-705 is through inhibition of influenza A virus RNA polymerase by the triphosphate metabolite (3). The compound did not affect cellular DNA or RNA synthesis, and inhibition of cellular IMP dehydrogenase was seen only at high dosage levels. These data prompted the present studies to ascertain the efficacy of T-705 against the avian influenza A (H5N1) virus both in vitro and in a mouse model. This report describes the effects of these treatments and also provides some background data on the influenza A/Duck/MN/1525/81 (duck/MN) (H5N1) virus used, since this is the first report of the use of this virus in animal studies.
MATERIALS AND METHODS
Compounds.
T-705 was provided by Y. Furuta of Toyama Chemical Co., Ltd. (Toyama, Japan). Oseltamivir carboxylate was obtained from C. Kim (Gilead Sciences, Foster City, CA). Zanamivir and oseltamivir were purchased from a local pharmacy. Ribavirin was provided by Z. Hong of Valeant Pharmaceuticals, Inc. (Costa Mesa, CA). T-705 was dissolved in dimethyl sulfoxide for in vitro studies and was suspended in 0.4% carboxymethyl cellulose (CMC) for animal studies. The other compounds were prepared in sterile minimum essential medium (MEM) or saline. The use of oseltamivir in animal studies took into account the excipient included in the purchased product. All preparations used in vivo were stored at 4°C until they were used.
Viruses and cells.
Influenza A/Duck/MN/1525/81 and A/Gull/PA/4175/83 (gull/PA) (H5N1) viruses were a gift from R. Webster, St. Jude Research Hospital (Memphis, TN). Influenza A/Hong Kong/213/2003 × Ann Arbor/6/60 and A/Vietnam/1203/04 × Ann Arbor/6/60 viruses were also used; these are attenuated hybrid viruses containing the A/Vietnam or A/Hong Kong hemagglutinin (HA) and NA, but with a core of the cold-adapted Ann Arbor virus, which contains the PB1, PB2, PA, NP, M, and NS gene segments of the latter virus generated by the use of reverse genetics. The HA gene was genetically modified to remove the stretch of basic amino acids connecting the HA1 and HA2 domains of HA, as described by Suguitan et al. (21). The latter viruses were obtained from George Kemble, MedImmune Vaccines, Inc. (Mountain View, CA). The viruses used in cell culture experiments were passaged through Madin-Darby canine kidney (MDCK) cells (American Type Culture Collection, Manassas, VA) at least once to prepare pools. The pools were then titrated in MDCK cells before use. The cells were grown in MEM containing 5% fetal bovine serum (HyClone Laboratories, Logan, UT) and 0.18% sodium bicarbonate with no antibiotics in a 5% CO2 incubator. Viral propagation and in vitro antiviral assays were run by using MDCK cells in MEM, 0.18% sodium bicarbonate, 10 units of trypsin/ml, 1 μg EDTA/ml, and 50 μg gentamicin/ml. All assays were incubated at 37°C.
The A/Duck virus was readily adapted to mice after two serial passages through the animals, and a pool was prepared in MDCK cells for use in the animal portions of these experiments. A description of the pathogenicity of this virus in mice is included in this report.
Animals.
Female specific-pathogen-free BALB/c mice weighing 18 to 21 g were obtained from Charles River Laboratories (Wilmington, MA). They were quarantined for 48 h prior to use and were fed standard mouse chow and tap water ad libitum. Animal procedures complied with the guidelines set forth by USDA and the Utah State University Institutional Animal Care and Use Committee.
SaO2 determinations.
Arterial oxygen saturation (SaO2) was determined with a Biox 3800 pulse oximeter (Ohmeda, Louisville, OH). The ear probe attachment was used, with the probe placed on the thigh of the mouse. Readings were made after 30 s of stabilization for each animal. This method has been described in detail (15). All SaO2 determinations were made on days 3 through 11 after virus exposure. The animals that died of obvious influenza virus infection during the experiment were assigned an SaO2 value of 75%, since the values normally do not drop below this level.
Lung virus titer determinations.
The mouse lungs were homogenized, and various dilutions of each were assayed in triplicate for infectious virus in MDCK cells grown in 96-well flat-bottom microplates, as described previously (19). Each lung homogenate was centrifuged at 2,000 × g for 5 min, and the supernatants were used in these assays.
In vitro antiviral evaluations.
Antiviral activity was determined in vitro by the following methods: inhibition of virus-induced cytopathic effect (CPE), as determined by visual (microscopic) examination of the cells; increases in neutral red dye uptake into cells; and virus yield reduction. These methods have been described previously (20). Eight concentrations of the test compounds, each of which varied by one-half log10 from the next concentration, were evaluated by use of MDCK cells. Standard placebo-treated virus controls, toxicity controls, and normal-medium controls were included in all assays. CPE inhibition data were expressed as the 50% effective (viral CPE-inhibitory) concentration (EC50); the 50% cytotoxic (cell-inhibitory) concentration (CC50); and the selectivity index (SI), which was determined as CC50/EC50. Virus yield reduction data were expressed as those concentrations that inhibited the virus yield by 1 log10 (EC90), as determined by regression analysis; the SI for virus yield results was calculated as CC50/EC90.
Studies of pathogenicity of A/Duck/MN/1525/81 (H5N1) virus in mice.
Mice were infected intranasally (i.n.) with a 100% lethal dose of the virus used in the following in vivo antiviral studies. This was equivalent to 105.5 50% cell culture infectious doses (CCID50s)/ml. A group of 20 mice was observed for death for 21 days; additional groups of 5 mice each were killed on days 1, 3, 5, and 7; and the lungs, spleens, kidneys, liver, and brains, as well as heparinized blood, were removed and assayed for the virus titer, as described above for lung virus. The lungs were also assigned a score ranging from 0 (normal) to 4 (maximum plum coloration), and all organs were weighed each time that they were taken.
In vivo antiviral studies.
Initial evaluation of the efficacy of T-705 was done by infecting groups of 19 mice i.n. with a 100% lethal dose of influenza A/Duck/MN/1525/81 (H5N1) virus. This was done by anesthetizing the mice by intraperitoneal injection of ketamine (100/mg/kg) and instilling 90 μl of 105.5 CCID50s/ml virus on the nares. The mice were treated per os (p.o.) with 33, 100, or 300 mg/kg of body weight/day of T-705 every 6 h for 5 days beginning 1 h after virus exposure or with 20 mg/kg/day of oseltamivir twice daily for 5 days beginning at the same time. This treatment schedule was identical to that used by Furuta et al. (4). As controls, 35 infected mice were treated with CMC in parallel with T-705. Parameters for determining the effects of treatment included the prevention of death through 21 days, lessening of the SaO2 decline, inhibition of lung consolidation (lung score and lung weight), and lessening of lung virus titers. The lung parameters were assayed on days 1, 3, and 6 of the infection, with three drug-treated and five placebo-treated mice killed at each time point. Ten mice were used per drug-treated group to assay for death rates and SaO2 levels. Toxicity controls were run in parallel at each drug dose; three mice were used per dose, with weights taken prior to the start of treatment and again 18 h after the last treatment, and the animals were observed for overt signs of toxicity and death for 21 days. Healthy controls were also included in each study; these animals were weighed along with the toxicity controls, and SaO2 levels were ascertained on the same days that they were ascertained for the infected animals. Three healthy controls were killed along with the infected mice to provide background lung data. On day 22 of the experiment, all surviving infected, treated mice and the healthy controls were rechallenged with the same influenza virus by use of a 100-fold higher virus concentration, and these animals were observed for death for 14 additional days. This experiment was repeated once to confirm the initial findings.
A further experiment was run to ascertain how long after virus exposure the four-times-daily treatment could be delayed and still provide significant antiviral effects. In this study, groups of 10 mice each were challenged with the same virus dose indicated above and treated with 300 mg/kg/day four times daily for 5 days beginning 24, 36, 48, and 60 h after virus exposure; the experiment was later repeated with treatments starting 72, 84, 96, or 120 h after virus exposure. Disease parameters included inhibition of death, prolongation of the mean day to death, and lessening of the SaO2 decline, as described above.
Three additional experiments were later run, with prevention or a delay in the time to death and inhibition of the SaO2 decline used as disease parameters. In these experiments, T-705 was administered once or twice daily for 5 days instead of four times daily or once at various times after virus exposure. In the experiments with the once- or twice-daily treatments, the doses used were expanded to include 300, 100, 30, 10, 3, and 1 mg/kg/day. In the single-treatment study, doses of only 600 and 300 mg/kg were used.
Statistical analysis.
The increases in the number of survivors were evaluated by chi-square analysis with Yates' correction. Differences in the mean day to death, mean SaO2 values, and mean lung virus titers were analyzed by the t test. The Wilcoxon ranked sum analysis test was used to compare lung scores.
RESULTS
Inhibition of influenza H5N1 viruses in vitro.
The results of the in vitro evaluation studies of the activities of T-705, oseltamivir carboxylate, zanamivir, and ribavirin against influenza A (H5N1) viruses are summarized in Table 1. At least two experiments were run with each virus, so the mean ± standard deviation (SD) could be determined. Overall, T-705 was efficacious against all of the viruses evaluated, with the mean EC50 values, determined by use of neutral red uptake, ranging from 2.6 to 12.1 μM. Visual determination of inhibition of viral CPE yielded very similar data, which are not shown. This efficacy was generally stronger than that seen with ribavirin but was considerably less than that seen with oseltamivir carboxylate and zanamivir. The virus yield reduction assay yielded generally similar results. Overall, the duck/MN and gull/PA viruses appeared to be more resistant to either of the neuraminidase inhibitors but were approximately equally sensitive to both T-705 and ribavirin.
TABLE 1.
In vitro inhibition of influenza A (H5N1) virus replication by T-705, oseltamivir carboxylate, zanamivir, and ribavirina
| Virus | Meanb neutral red EC50 (μM) ± SD
|
Virus yield reduction EC90 (μM) ± SD
|
||||||
|---|---|---|---|---|---|---|---|---|
| T-705 | Oseltamivir carboxylate | Zanamivir | Ribavirin | T-705 | Oseltamivir carboxylate | Zanamivir | Ribavirin | |
| A/Hong Kong/213/03 × Ann Arbor/6/60 | 2.6 ± 1.3 | 0.032 ± 0.018 | 0.09 ± 0.09 | 14.3 ± 5.3 | 1.3 ± 0.6 | 0.07 ± 0.04 | 0.42 ± 0.33 | 33.2 ± 22.5 |
| A/Vietnam/1203/04 × Ann Arbor/6/60 | 12.1 ± 17.3 | 0.0014 ± 0.0007 | 0.012 ± 0.015 | 35.7 ± 15.2 | 3.8 ± 0.6 | 0.007 ± 0.007 | 0.09 ± 0.06 | 18.4 ± 9.4 |
| A/Duck/MN/1525/81 | 4.5 ± 3.8 | 0.14 ± 0.21 | 0.15 ± 0.06 | 43.0 ± 18.4 | 2.6 ± 0.6 | 0.92 ± 1.3 | 0.96 ± 1.32 | 20.1 ± 12.3 |
| A/Gull/PA/4175/83 | 11.5 ± 10.9 | 0.28 ± 0.07 | 0.15 ± 0.09 | 38.1 ± 2.5 | 7.7 ± 4.2 | 0.67 ± 0.21 | 1.23 ± 2.07 | 32.0 ± 26.2 |
The CC50s for each compound were as follows: T-705, >641 μM; oseltamivir carboxylate, >3,521 μM; zanamivir, >3,612 μM; ribavirin, 2,336 ± 943 μM.
Results of two to five experiments with each compound.
Pathogenicity of A/Duck/MN/1525/81 (H5N1) virus in mice.
All mice infected with the A/Duck/MN/1525/81 (H5N1) virus died by day 8, with a mean time to death of 6.6 ± 0.8 days. Lung consolidation, as manifested by nearly maximal lung scores (plum coloration with a mean of 3.5), and a mean lung weight of 310 mg compared to a mean lung weight of 135 mg for the healthy controls were seen by day 5, the day on which the animals began to die. Viral replication in the mice following exposure to the A/Duck/MN/1525/81 (H5N1) virus is summarized in Table 2. Mean virus titers ranging from 5.5 to 6.3 log10 CCID50/ml were obtained from the lungs at all times that the animals were killed; on days 1 and 3, one of the five plasma samples assayed had detectable virus, but in that sample the virus titer was relatively high (5.8 to 6.3 log10 CCID50/ml); on day 5, plasma from four of the five infected mice had detectable virus, and the mean titer was 5.3 log10 CCID50/ml. The virus appeared to be mildly neurotropic, with one of the five brains taken on days 1 and 5 exhibiting virus at very low titers (1.75 to 2.5 log10 CCID50/ml). No virus could be detected in the kidney, liver, or spleen. The weights of the livers, spleens, and kidneys taken during the experiment declined during the study; but the brain weights remained relatively similar throughout the study (data not shown).
TABLE 2.
Kinetics of replication of influenza A/Duck/MN/525/81 (H5N1) virus in mouse tissuesa
| Day after virus exposure | Mean virus titerb (no. of samplesc positive/total no. tested)
|
|||||
|---|---|---|---|---|---|---|
| Lungs | Plasma | Brain | Kidney | Liver | Spleen | |
| 1 | 6.3 ± 0.3 (5/5) | 5.8 (1/5) | 2.5 (1/5) | <1.75 (0/5) | <1.75 (0/5) | <1.75 (0/5) |
| 3 | 5.9 ± 0.3 (5/5) | 6.3 (1/5) | <1.75 (0/5) | <1.75 (0/5) | <1.75 (0/5) | <1.75 (0/5) |
| 5 | 6.1 ± 0.3 (5/5) | 5.3 ± 1.4 (4/5) | <1.75 (1/5) | <1.75 (0/5) | <1.75 (0/5) | <1.75 (0/5) |
| 7 | 5.5 ± 0.0 (1/1) | <1.75 (0/1) | 1.75 (1/1) | <1.75 (0/1) | <1.75 (0/1) | <1.75 (0/1) |
The virus challenge dose was 105.5 CCID50/ml.
The titer of tissue or plasma yielding detectable virus (one experiment with five mice per time point).
The tissue or plasma sample from which virus was detected (limit of method, 101.75 CCID50/g).
Efficacies of multiple T-705 treatments on lethal influenza virus infections in mice.
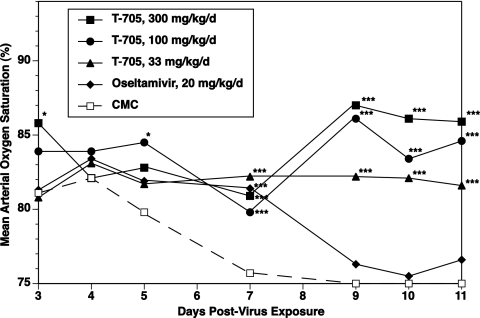
In the two experiments run by using four-times-daily T-705 treatments, the virus infection was markedly inhibited, with essentially all infected, treated mice surviving the duration of the study (Table 3). The animals displayed significantly lessened SaO2 declines (data from experiment 2 are shown in Fig. 1) and had significantly lessened lung scores, inhibited lung weight increases, and reduced lung virus titers (Table 3). Oseltamivir was less efficacious, preventing deaths in only 10 to 20% of the infected mice, although the treatment significantly delayed the mean day to death in each experiment (Table 3). The other disease parameters were inhibited to a lesser degree by oseltamivir than by T-705. Rechallenge of the surviving infected mice with a 100-fold higher concentration than that used initially was fully lethal for the healthy control mice, but all infected animals treated with T-705 survived the second virus challenge. The two surviving oseltamivir-treated mice in the first experiment succumbed to this rechallenge, but the single animal in the oseltamivir-treated group which was alive in the second experiment also survived the rechallenge.
TABLE 3.
Effect of four-times-daily treatmenta with T-705 or oseltamivir on an infection in mice induced by influenza A/Duck/MN/1525/81 (H5N1) virus
| Expt no. and treatmenta | Dose (mg/kg/day) | Infected, treated miceg
|
Toxicity controls
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of survivors/total no. tested | MDDb ± SD | Day 1 mean lung virus titerc ± SD | Day 6 mean lung scored ± SD | Day 6 mean lung wt (mg) ± SD | Mean day 11 SaO2 (%) ± SD | No. of survivors/total no. tested following rechallengee | No. of survivors/total no. tested | Mean host wt changef (g) | ||
| Expt 1 | ||||||||||
| T-705 | 300 | 10/10*** | >21.0*** | 3.5 ± 0.8*** | 0.3 ± 0.3* | 146 ± 12*** | 88.6 ± 3.8*** | 10/10 | 3/3 | 0.6 |
| T-705 | 100 | 10/10*** | >21.0*** | 4.9 ± 0.3** | 0.5 ± 0.0* | 140 ± 20*** | 85.9 ± 5.9*** | 10/10 | 3/3 | 1.1 |
| T-705 | 33 | 10/10*** | >21.0*** | 5.8 ± 0.5 | 1.3 ± 0.3* | 217 ± 6** | 84.8 ± 4.8*** | 10/10 | 3/3 | 0.2 |
| Oseltamivir | 20 | 2/10 | 9.4 ± 1.2*** | 6.1 ± 0.5 | 2.2 ± 0.6* | 270 ± 36* | 76.9 ± 3.8* | 0/2 | 3/3 | 0.6 |
| CMC | 0/20 | 6.6 ± 1.1 | 6.3 ± 0.4 | 3.3 ± 0.3 | 360 ± 42 | 75.0 ± 0.0 | ||||
| Healthy controls | 0.0 ± 0.0 | 0.0 ± 0.0 | 117 ± 21 | 92.6 ± 3.5 | 0/5 | 3/3 | 0.8 | |||
| Expt 2 | ||||||||||
| T-705 | 300 | 9/9*** | >21.0*** | 2.4 ± 0.4*** | 0.5 ± 0.0* | 123 ± 15*** | 85.9 ± 6.9*** | 9/9 | 3/3 | 0.0 |
| T-705 | 100 | 10/10*** | >21.0*** | 5.3 ± 0.3** | 0.7 ± 0.3* | 153 ± 15*** | 84.6 ± 4.4*** | 10/10 | 3/3 | −0.1 |
| T-705 | 33 | 9/10*** | 12.0 ± 0.0 | 5.8 ± 0.4 | 1.7 ± 0.6* | 173 ± 6*** | 81.6 ± 6.3*** | 9/9 | 3/3 | 0.0 |
| Oseltamivir | 20 | 1/10 | 9.3 ± 1.7*** | 6.3 ± 0.1 | 3.5 ± 0.0 | 247 ± 23** | 76.6 ± 3.9 | 1/1 | 3/3 | 0.2 |
| CMC | 0/20 | 6.1 ± 0.8 | 6.4 ± 0.4 | 3.6 ± 0.2 | 328 ± 13 | 75.0 ± 0.0 | ||||
| Healthy controls | 5/5 | >21.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 117 ± 6 | 93.2 ± 2.8 | 0/5 | 3/3 | 1.3 | |
The doses were administered every 6 h for 5 days beginning 1 h after virus exposure (oseltamivir was given twice daily for 5 days beginning 1 h after virus exposure).
MDD, mean day to death of mice dying prior to day 21.
Log10 CCID50/g.
Plum coloration of lung ranging from 0 (normal lung) to 4 (maximal coloration).
Rechallenged with 100-fold more virus on day 21.
Difference between initial weight and weight 18 h after final treatment.
*, P < 0.05 compared to the results for the CMC-treated controls; **, P < 0.01 compared to the results for the CMC-treated controls; ***, P < 0.001 compared to the results for the CMC-treated controls.
FIG. 1.
Effect of four-times-daily treatment with T-705 or twice-daily treatment with oseltamivir beginning 1 h after virus exposure on arterial oxygen saturation decline in BALB/c mice infected with influenza A/Duck/MN/1525/81 (H5N1) virus. Data are expressed as the mean values for 10 mice per time point. *, P < 0.05 compared to the results for the CMC-treated controls; **, P < 0.01 compared to the results for the CMC-treated controls; ***, P < 0.001 compared to the results for the CMC-treated controls.
Reducing the number of T-705 treatments to once or twice daily did not affect the influenza virus-inhibitory effects of the compound (Table 4), with doses down to 30 mg/kg/day significantly preventing death and lessening the SaO2 decline of the infected mice. It should be noted that the infection in the once-daily study was lethal for only 65% of the placebo-treated animals.
TABLE 4.
Effect of once- or twice-daily T-705 treatment on an influenza A (H5N1) virus infection in mice
| Expt no. and Treatmenta | Dosage (mg/kg/day) | Toxicity controls
|
Infected, treated mice
|
|||
|---|---|---|---|---|---|---|
| No. of survivors/total no. tested | Mean host wt changeb (g) | No. of survivors/total no. testedd | MDDc ± SDd | Mean day 11 SaO2 (%) ± SDd | ||
| Expt 1 | ||||||
| T-705, once daily | 300 | 3/3 | 0.4 | 10/10*** | >21.0*** | 82.7 ± 2.4*** |
| T-705, once daily | 100 | 3/3 | 0.4 | 10/10*** | >21.0*** | 80.8 ± 2.0*** |
| T-705, once daily | 30 | 3/3 | 0.7 | 9/10** | 7.0 ± 0.0 | 81.4 ± 4.7*** |
| T-705, once daily | 10 | 3/3 | 0.6 | 4/10 | 7.3 ± 0.8 | 77.0 ± 2.8 |
| T-705, once daily | 3 | 3/3 | 0.3 | 0/10 | 7.0 ± 0.5 | 75.0 ± 0.0 |
| 0.4% CMC | 7/20 | 7.5 ± 1.1 | 76.7 ± 2.7 | |||
| Healthy controls | 5/5 | 1.2 | 86.0 ± 3.8 | |||
| Expt 2 | ||||||
| T-705, twice daily | 300 | 3/3 | 0.2 | 10/10*** | >21.0*** | 86.9 ± 2.8*** |
| T-705, twice daily | 100 | 3/3 | 1.0 | 10/10*** | >21.0*** | 84.2 ± 1.3*** |
| T-705, twice daily | 30 | 3/3 | 0.1 | 8/10*** | 11.0 ± 4.2 | 79.8 ± 4.4*** |
| T-705, twice daily | 10 | 3/3 | 0.1 | 2/10 | 8.5 ± 2.8 | 75.5 ± 1.0* |
| T-705, twice daily | 3 | 3/3 | 0.8 | 0/10 | 7.4 ± 1.1 | 75.0 ± 0.0 |
| 0.4% CMC | 0/20 | 8.2 ± 1.6 | 75.0 ± 0.0 | |||
| Healthy controls | 5/5 | 0.5 | 85.6 ± 3.6 | |||
Five-day treatments beginning 1 h after virus exposure.
Difference between initial weight and weight 18 h after final treatment.
MDD, mean day to death of mice dying prior to day 21.
*, P < 0.05 compared to the results for the CMC-treated controls; **, P < 0.01 compared to the results for the CMC-treated controls; ***, P < 0.001 compared to the results for the CMC-treated controls.
T-705 and oseltamivir appeared to be well tolerated in the toxicity control mice used in parallel in these studies, as seen by no deaths and no weight loss during the time of treatment (Tables 3 and 4).
Effects of delayed initiation of four-times-daily T-705 treatment on lethal influenza virus infection.
Two experiments were run to determine the effect of a delay in the start of the four-times-daily T-705 treatments on this avian influenza virus infection. In the first experiment, treatments were delayed to 60 h after virus exposure. In the second experiment, the treatment initiation was delayed to 72, 84, 96, or 120 h after virus exposure. The results of both experiments are summarized in Table 5. The prevention of death and lessening of the day 11 SaO2 decline were highly significant for each treatment initiation time out to 96 h after virus exposure, and 30% (P < 0.05) of the infected mice also survived when therapy began 120 h (5 days) after viral challenge.
TABLE 5.
Effect of delay in initiation of T-705 treatment on an influenza A (H5N1) virus infection in mice
| Expt no. and treatmenta | Time of start of therapy (h) | Infected, treated mice
|
||
|---|---|---|---|---|
| No. of survivors/total no. tested | MDDb ± SDc | Mean day 11 SaO2 (%) ± SDc | ||
| Expt 1 | ||||
| T-705, 300 mg/kg/day | 24 | 10/10*** | >21.0*** | 85.3 ± 5.7*** |
| T-705, 300 mg/kg/day | 36 | 9/9*** | >21.0*** | 84.6 ± 5.9*** |
| T-705, 300 mg/kg/day | 48 | 9/10*** | 4.0 ± 0.0 | 87.1 ± 6.2*** |
| T-705, 300 mg/kg/day | 60 | 10/10*** | >21.0*** | 84.6 ± 6.6*** |
| 0.4% CMC | 24 | 0/20 | 7.0 ± 0.5 | 75.0 ± 0.0 |
| Healthy controls | 92.6 ± 3.5 | |||
| Expt 2 | ||||
| T-705, 300 mg/kg/day | 72 | 9/10*** | 7.0 ± 0.0 | 84.1 ± 5.5*** |
| T-705, 300 mg/kg/day | 84 | 10/10*** | >21.0*** | 81.7 ± 3.6** |
| T-705, 300 mg/kg/day | 96 | 8/9*** | 7.0 ± 0.0 | 81.2 ± 3.4** |
| T-705, 300 mg/kg/day | 120 | 3/10* | 7.9 ± 2.6 | 76.0 ± 1.3 |
| 0.4% CMC | 72 | 0/20 | 7.9 ± 2.7 | 75.1 ± 0.5 |
| Healthy controls | 86.4 ± 3.4 | |||
Every 6 h for 5 days.
MDD, mean day to death of mice dying prior to day 21.
*, P < 0.05 compared to the results for the CMC-treated controls; **, P < 0.01 compared to the results for the CMC-treated controls; ***, P < 0.001 compared to the results for the CMC-treated controls.
Effects of delayed initiation of single T-705 treatment on lethal influenza virus infection.
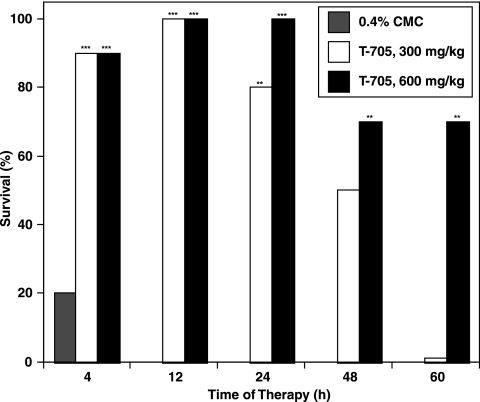
The results presented above, which indicate that even a single daily treatment with T-705 was efficacious against this avian influenza virus infection, prompted a final experiment in which the compound was administered a single time to mice infected with the virus. It was anticipated that such a treatment may be efficacious only if it was applied at the most appropriate time during the virus infection, so single treatments with 600 or 300 mg/kg of the compound were administered 4, 12, 24, 48, or 60 h after virus exposure. The results, summarized in Fig. 2, indicate that both dosages of T-705 were highly protective for the infected mice when they were given at all times up to 48 h after virus exposure. Only the high dose was inhibitory at the 60-h treatment time. In this experiment, the infection in the CMC-treated controls was lethal for 80% of the mice. T-705 was well tolerated at both dosages studied, with all toxicity control mice surviving and gaining weight during the experiment (data not shown).
FIG. 2.
Effect of single T-705 treatment at various times after virus exposure on prevention of death in mice infected with influenza A A/Duck/MN/1525/81 (H5N1) virus. *, P < 0.05 compared to the results for the carboxymethyl cellulose-treated controls (CMC); **, P < 0.01 compared to the results for the carboxymethyl cellulose-treated controls (CMC); ***, P < 0.001 compared to the results for the CMC-treated controls.
DISCUSSION
The A/Duck/MN/1525/81 (H5N1) virus used in this study was described by Matrosovich et al. (12), who compared its ability to bind to the sialic acid-α-2-3galactose (Sia2-3Gal) and Sia2-6Gal receptors of other viruses from aquatic birds as well as the human A/HK/156/97 (H5N1) virus isolate. The association constant for the duck/MN virus to the Sia2-3Gal receptor was 3.3 U−1, which was relatively high; the constant for the human isolate was 1.5 U−1, indicating some differences between the two viruses. Neither virus had a detectable association constant for the Sia2-6Gal receptor, which confirms reports that the human influenza viruses prefer the Sia2-6Gal receptors and that the avian viruses favor those receptors terminating in Sia2-3Gal (1); the data also indicate that the human H5N1 virus isolate still carries the avian receptor-binding phenotype. It is apparent that mice have a preponderance of the Sia2-3Gal receptors (F. Fang [NexBio Pharmaceuticals, Inc.], personal communication), which would explain why the duck/MN virus used in the current studies adapted so readily to mice. The duck/MN virus was relatively less virulent than the A/Vietnam/1203/04 or the A/Hong Kong/156/97 (H5N1) viruses, requiring a viral challenge several log10 higher to be lethal to the mice. The H5N1 viruses can be avirulent or highly virulent in poultry, depending on a number of basic amino acid residues that connect the HA1 and HA2 subunits. The duck/MN virus, which is less virulent in birds, lacks a stretch of basic amino acids between HA1 and HA2; it would be expected that an avian virus with such a low virulence may be less neurotropic than the highly pathogenic A/Hong Kong or A/Vietnam (H5N1) viruses reported by others (11, 24). The duck/MN virus was used because it, as well as the gull/PA and the hybrid viruses used in vitro, were exempted from the list of USDA high-consequence livestock pathogens (USDA, personal communication). Further studies will be needed to determine how well the efficacy of T-705 against the duck/MN virus used in these studies will predict efficacy against the recent human isolates of the H5N1 virus.
The substituted pyrazine T-705 exhibited significant inhibitory effects against all the H5N1 influenza viruses used in our studies. The antiviral potency of the compound, as expressed by EC50 and EC90 values, was in the range previously reported by Furuta et al. (4) and Takahashi et al. (23) in their studies with other influenza virus serotypes. It should be pointed out, however, that this compound is rather insoluble in aqueous medium, and this may have affected its potency in vitro. The EC50 and EC90 values of oseltamivir carboxylate and zanamivir were generally at least 1 to 2 log10 lower, particularly against the hybrid H5N1 viruses used in the present experiments, than those that have been reported against other influenza A serotypes (16, 20). Ribavirin's efficacy was similar to what we have reported previously for the duck and gull viruses and for other influenza virus serotypes as well (17).
The in vivo experiments described in this report indicate that oral therapy with T-705 was highly efficacious against experimental infections in mice induced by the duck, H5N1 influenza virus. Indeed, the efficacy of T-705 against this H5N1 virus seen at 33 mg/kg/day exceeded that reported by Furuta et al. (4), who found that T-705 offered only weak protection against the A/PR/8/34 (H1N1) virus when it was used at 50 mg/kg/day. The four-times-daily treatment schedule initially used was the same schedule reported by Furuta et al. (4), who found that an influenza A (H1N1) virus infection was significantly inhibited in mice. This schedule was originally selected by that group on the basis of pharmacokinetic data which showed that the drug cleared the plasma of orally treated mice by 90% within 6 h after treatment (Toyama Chemical Co., Ltd., unpublished data). Since such a treatment schedule is not ideal, schedules with once- or twice-daily p.o. treatments were also evaluated, and strong efficacy against the avian influenza virus infection was again seen; these results prompted the study with once-only therapy, in which strong inhibitory effects against the infection in mice were still apparent. These data suggest that the compound may persist in cells longer than it persisted in plasma; studies are under way to investigate this persistence of T-705 in cells. As indicated earlier, Furuta et al. (3) reported that T-705 appears to act by specific inhibition of the influenza virus RNA polymerase by the triphosphorylated metabolite of the compound. The observation that the four-times-daily treatments could be delayed to start until 96 h after virus exposure, at a time when lung virus titers are already high and lung consolidation is approaching maximal levels, with a resultant decrease in SaO2 levels, strongly indicates the potential for T-705 to be used therapeutically for the control of influenza virus infections. It is notable that ribavirin, also known to be a specific inhibitor of influenza virus RNA polymerase at the metabolized triphosphate level (2), has been shown to inhibit influenza virus infections in mice when treatment initiation was delayed to at least 48 h (17), which suggests that this common mechanism of action will allow late starts in therapy of influenza virus infections.
The rechallenge studies run with a portion of the experiments described here were undertaken to determine if the initial infection had been sufficient to induce complete immunity against the influenza virus infection in these mice, despite the striking inhibition of the infection by the antiviral treatments. The data, which showed that the animals survived the challenge, indicate that satisfactory immunity did indeed develop.
Oseltamivir did not exert the strongly positive in vivo effects against this avian influenza virus in the present studies that have been seen against other influenza virus subtypes (13, 18). This lesser activity of oseltamivir against avian influenza virus infections in mice has also been reported by Yen et al. (27). In their experiments with mice infected with the highly pathogenic A/Vietnam/1203/04 (H5N1) virus, oseltamivir was very weakly effective at a dose of 10 mg/kg/day administered p.o. twice daily for 5 days beginning 4 h before virus exposure. It was found that if the therapy was extended to 8 days, this same dosage provided a more protective effect, although 20% of the infected mice still died. Influenza A (H5N1) virus neuraminidase inhibition by these compounds has not been extensively reported, although Govorkova et al. (5) showed that oseltamivir carboxylate is generally less effective in inhibiting this enzyme activity than either zanamivir or peramivir (RWJ-270201). In the present study, it should also be noted that the oseltamivir treatment of the duck influenza virus infection began 1 h after virus exposure. This may also affect the drug's ability to inhibit the infection, although we have previously reported that therapy begun as late as 60 h after challenge with an influenza A (H1N1) virus still rendered a strongly protective effect in mice (18). Thus, this neuraminidase inhibitor may not be ideal for therapy of avian influenza virus infections. T-705 did not exhibit any signs of toxicity in the present series of experiments, with all control mice surviving at all dosages used and with weight gain generally seen. We have done further studies with single p.o. treatments with T-705, using dosages as high as 1,200 mg/kg, with the compound well tolerated even at that high dose (data not shown).
Overall, these data obtained by use of the mouse model indicate the potential utility of the pyrazine derivative T-705 for the treatment of avian influenza virus infections in the clinic. It would be appropriate to also use the ferret influenza virus infection model for further evaluation of the compound. The possibility also exists that T-705 may be synergistically effective if it is used with another influenza virus inhibitor with a different mechanism of action. Studies are under way to consider such synergism. The compound is currently undergoing extensive toxicology and pharmacology studies in order to begin clinical trials.
Acknowledgments
This work was supported by contracts NO1-AI-15435 and NO1-AI-30048 from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Appreciation is expressed to George Kemble of MedImmune Vaccines, Inc., for providing the attenuated viruses used in the in vitro evaluations described in this report.
Footnotes
Published ahead of print on 28 December 2006.
REFERENCES
- 1.Baigent, S. J., and J. W. McCauley. 2003. Influenza type A in humans, mammals and birds: determinants of virus virulence, host-range and interspecies transmission. Bioessays 25:657-671. [DOI] [PubMed] [Google Scholar]
- 2.Eriksson, B., E. Helgstrand, N. G. Johannson, A. Larsson, A. Misiorny, J. O. Noren, L. Philipson, K. Stenburg, G. Stenning, S. Stridh, and B. Oberg. 1977. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob. Agents Chemother. 11:946-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuta, Y., K. Takahashi, M. Kuno-Maikawa, H. Sangawa, S. Uehara, K. Kozaki, N. Nomura, H. Egawa, and K. Shiraki. 2005. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 49:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuta, Y., K. Takahashi, Y. Fukuda, M. Kuno, T. Kamiyama, K. Kozaki, N. Nomura, H. Egawa, S. Minami, Y. Watanabe, H. Narita, and K. Shiraki. 2002. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 46:977-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Govorkova, E. A., I. A. Leneva, O. G. Goloubeva, K. Bush, and R. G. Webster. 2001. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob. Agents Chemother. 45:2723-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubareva, L. V., J. A. McCullers, R. C. Bethell, and R. G. Webster. 1998. Characterization of influenza A/HongKong/156/97 (H5N1) virus in a mouse model and protective effect of zanamivir on H5N1 infection in mice. J. Infect. Dis. 178:1592-1596. [DOI] [PubMed] [Google Scholar]
- 7.Hayden, F., A. Klimov, M. Tashiro, A. Hay, A. Monto, J. McKimm-Breschkin, C. Macken, A. Hampson, R. G. Webster, M. Amyard, and M. Zambon. 2005. Neuraminidase inhibitor susceptibility network position statement: antiviral resistance in influenza A/H5N1 viruses. Antivir. Ther. 10:873-877. [PubMed] [Google Scholar]
- 8.Kaye, D., and C. R. Pringle. 2005. Avian influenza viruses and their implication for human health. Clin. Infect. Dis. 40:108-112. [DOI] [PubMed] [Google Scholar]
- 9.Lemon, S. M., and A. A. F. Mahoud. 2005. The threat of pandemic influenza: are we ready? Biosecur. Bioterrorism Biodef. Strategy Pract. Sci. 3:70-73. [DOI] [PubMed] [Google Scholar]
- 10.Leneva, I. A., O. Goloubeva, R. J. Fenton, M. Tisdale, and R. G. Webster. 2001. Efficacy of zanamivir against avian influenza A viruses that possess genes encoding H5N1 internal proteins and are pathogenic in mammals. Antimicrob. Agents Chemother. 45:1216-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maines, T. R., X. H. Lu, S. M. Erb, L. Edwards, J. Guarner, P. W. Greer, D. C. Nguyen, K. J. Szretter, L.-M. Chen, P. Thawatsupha, M. Chittaganpitch, S. Waicharoen, D. T. Nguyen, T. Nguyen, H. H. T. Nguyen, J.-H. Kim, L. T. Hoang, C. Kang, L. S. Phuong, W. Lim, S. Zaki, R. O. Donis, N. J. Cox, J. M. Katz, and T. M. Tumpey. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 79:11788-11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matrosovich, M., N. Zhou, Y. Kawaoka, and R. Webster. 1999. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 73:1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendel, D., C. Y. Tai, P. A. Escarpe, W. Li, R. W. Sidwell, J. H. Huffman, C. Sweet, K. J. Jakeman, J. Merson, S. A. Lacy, W. Lew, M. A. Williams, L. Zhang, M. S. Chen, N. Bischofberger, and C. U. Kim. 1998. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS4071 protects mice and ferrets against influenza infection. Antimicrob. Agents Chemother. 42:640-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puthavathana, P., P. Auewarakul, P. C. Charoenying, K. Sangsiriwut, P. Pooruk, K. Boonnak, R. Kanyok, P. Thauachsupa, R. Kijphati, and P. Sawanpanyalert. 2005. Molecular characterization of the complete genome of human influenza H5N1 virus isolates from Thailand. J. Gen. Virol. 86:423-433. [DOI] [PubMed] [Google Scholar]
- 15.Sidwell, R., J. Huffman, J. Gilbert, B. Moscon, G. Pedersen, R. Burger, and R. Warren. 1992. Utilization of pulse oximetry for the study of the inhibitory effects of antiviral agents on influenza virus in mice. Antimicrob. Agents Chemother. 36:473-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidwell, R. W., and D. F. Smee. 2002. Peramivir (BCX-1812, RWJ-270201): potential new therapy for influenza. Expert Opin. 11:859-869. [DOI] [PubMed] [Google Scholar]
- 17.Sidwell, R. W., K. W. Bailey, M. H. Wong, D. L. Barnard, and D. F. Smee. 2005. In vitro and in vivo influenza virus-inhibitory effects of viramidine. Antivir. Res. 68:10-17. [DOI] [PubMed] [Google Scholar]
- 18.Sidwell, R. W., J. H. Huffman, D. L. Barnard, K. W. Bailey, M. H. Wong, A. Morrison, T. Syndergaard, and C. U. Kim. 1998. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antivir. Res. 37:107-120. [DOI] [PubMed] [Google Scholar]
- 19.Sidwell, R. W., D. F. Smee, J. H. Huffman, D. L. Barnard, K. W. Bailey, J. D. Morrey, and Y. S. Babu. 2001. In vivo influenza-inhibitory effects of the cyclopentane neuraminidase inhibitor RWJ-270201. Antimicrob. Agents Chemother. 45:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smee, D. F., J. H. Huffman, A. C. Morrison, D. L. Barnard, and R. W. Sidwell. 2001. Cyclopentane neuraminidase inhibitors with potent in vitro anti-influenza virus activity. Antimicrob. Agents Chemother. 45:743-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suguitan, A. L., J. McAuliffe, K. L. Mills, H. Jin, G. Duke, B. Lu, C. J. Luke, B. Murphy, D. E. Swayne, G. Kemble, and K. Subbarao. 2006. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 3:e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweeny, D. J., G. Lynch, A. M. Bidgood, W. Lew, K.-Y. Wang, and K. C. Cundy. 2000. Metabolism of the influenza neuraminidase inhibitor prodrug oseltamivir in the rat. Drug Metab. Dispos. 28:737-741. [PubMed] [Google Scholar]
- 23.Takahashi, K., Y. Furuta, Y. Fukuda, M. Kuno, T. Kamiyama, K. Kozaki, N. Nomura, H. Egawa, S. Minami, and K. Shiraki. 2003. In vitro and in vivo activities of T-705 and oseltamivir against influenza virus. Antivir. Chem. Chemother. 14:235-241. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka, H., C. H. Park, A. Ninomiya, H. Ozaki, A. Takada, and H. Kida. 2003. Neurotropism of the 1997 Hong Kong H5N1 influenza virus in mice. Vet. Microbiol. 95:1-13. [DOI] [PubMed] [Google Scholar]
- 25.Trampuz, A., R. M. Prabhu, T. F. Smith, and L. M. Beddour. 2004. Avian influenza: a new pandemic threat? Mayo Clin. Proc. 79:523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO Writing Committee. 2005. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353:1374-1385. [DOI] [PubMed] [Google Scholar]
- 27.Yen, H. L., A. S. Monto, R. G. Webster, and E. A. Govorkova. 2005. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J. Infect. Dis. 192:665-672. [DOI] [PubMed] [Google Scholar]