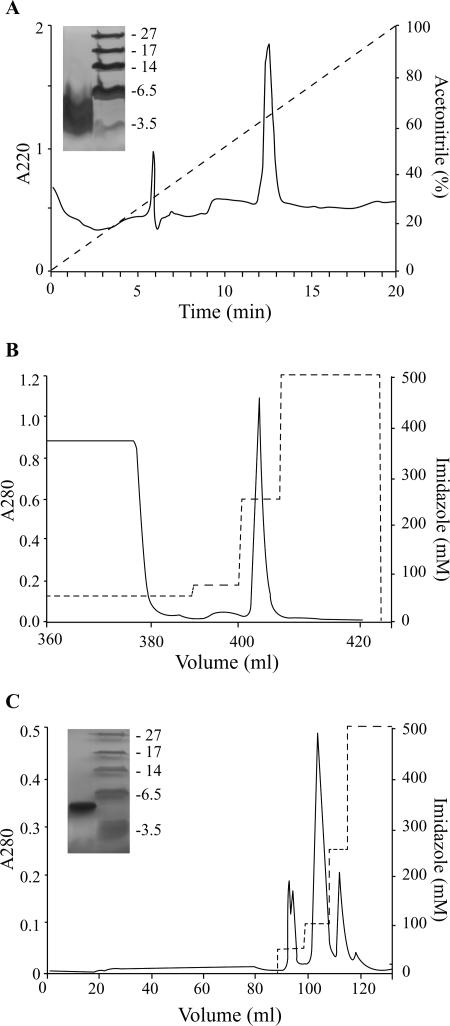
FIG. 4.
Purification of synthetic and recombinant Gal-6. (A) Synthetic peptide was loaded on a C8 RP-HPLC column and eluted with a 20-min linear 0 to 100% gradient of acetonitrile in 0.1% trifluoroacetic acid at a 1-ml/min flow rate. Peaks were detected at 220 nm. The purity of synthetic Gal-6 was verified on a Tricine-SDS-PAGE gel. MALDI-TOF analysis showed a monoisotopic mass of 4,282 ± 4 Da, in accordance with the calculated mass. (B) Fusion protein HGH-His8-TEV-rGal-6 was purified from cell supernatant by affinity chromatography. Bound fusion protein was eluted from the HisTrap nickel column with 250 mM imidazole in buffer A (25 mM Tris, 300 mM NaCl, pH 8.2). (C) Dialyzed fusion protein was proteolytically cleaved by TEV protease for 2 h at 30°C and reapplied to the nickel affinity column. The cleaved mature recombinant gallinacin-6 was eluted from the column at 50 mM imidazole in buffer A. The purity of rGal-6 was verified by Tricine-SDS-PAGE. The molecular mass of 4,313 ± 4 Da, determined by MALDI-TOF analysis, was in accordance with the calculated mass.