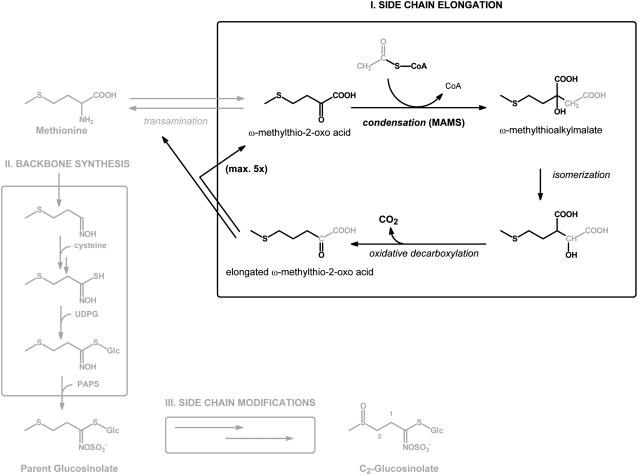
Figure 2.
General scheme for the biosynthesis of aliphatic glucosinolates involving Met side-chain elongation (I), backbone synthesis (II), and side-chain modifications (III). The proposed cycle for side-chain elongation of deaminated Met (I) commences with an aldol-type condensation between the respective ω-methylthio-2-oxo acid and acetyl-CoA, a reaction catalyzed by MAM. The methylthioalkylmalate product is converted through subsequent isomerization and oxidative decarboxylation into a ω-methylthio-2-oxo acid that is elongated by one methylene group. The elongated ω-methylthio-2-oxo acid is either transaminated and enters glucosinolate backbone synthesis (II) or undergoes additional cycles of side-chain elongation. Glucosinolate backbone synthesis is represented in a simplified manner without the identified enzymes and cofactors. The depicted end product, 2-methylsulfinylethyl glucosinolate, is a C2-glucosinolate that has not been side-chain elongated and does not occur naturally in Arabidopsis.