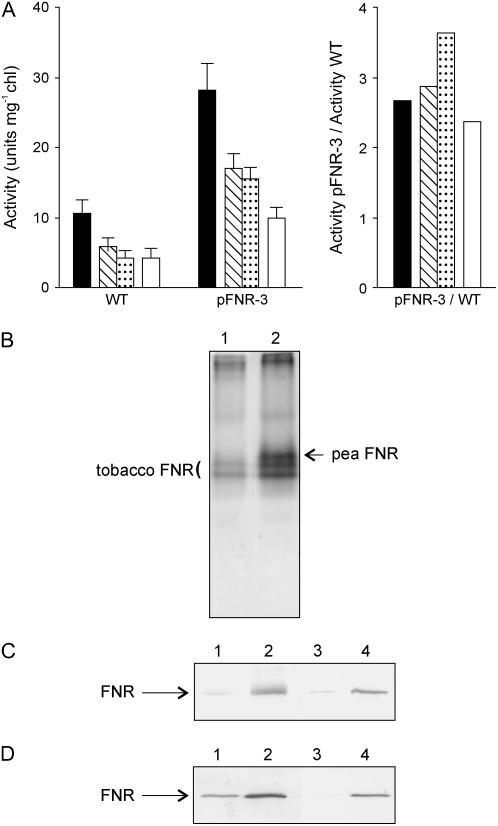
Figure 2.
Activity and subcellular localization of FNR in transgenic plants. A, FNR activity in thylakoids and stromal extracts of wild-type and FNR-expressing plants. Intact chloroplasts were isolated from 6-week-old wild-type and pFNR-3 plants and osmotically shocked. Stroma was separated by centrifugation and the resulting thylakoid pellet washed four times in hypotonic medium. NADPH-ferricyanide diaphorase activity was measured in whole broken chloroplasts (black bars), thylakoids washed two (hatched bars) or four (dotted bars) times, and stroma (white bars). The activity ratio represents the increase of diaphorase activity in each fraction of pFNR-3 relative to wild-type chloroplasts. B, Electrophoretic mobility of pea-derived FNR during nondenaturing PAGE of tobacco leaf extracts. Cleared lysates from wild-type (lane 1) and pFNR-3 leaves (lane 2), corresponding to 20 μg of total soluble protein, were resolved by native electrophoresis and stained for diaphorase activity. Tobacco and pea FNR isoforms are indicated. C, Subcellular localization of FNR in pFNR-3 plants. Total leaf extracts (lanes 1 and 2) and isolated chloroplasts (lanes 3 and 4) from 6-week-old wild-type (lanes 1 and 3) or pFNR-3 (lanes 2 and 4) plants were fractionated by SDS-PAGE and blotted onto nitrocellulose membranes for immunodetection of FNR. Samples corresponding to 5 μg chl were loaded onto each lane. D, Suborganellar localization of FNR. Thylakoids (lanes 1 and 2) and stroma (lanes 3 and 4) were separated after osmotic shock of isolated intact chloroplasts from wild-type (lanes 1 and 3) and pFNR-3 (lanes 2 and 4) plants. Samples corresponding to 5 μg chl were resolved by SDS-PAGE and the presence of FNR was determined by immunoblot analysis.