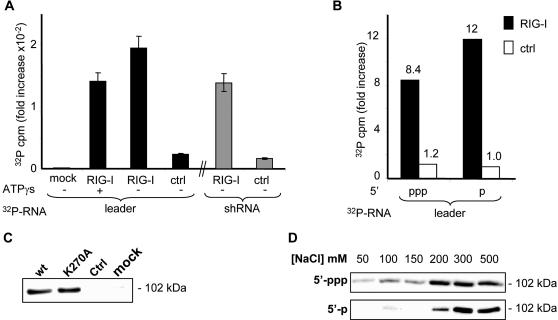
Figure 4. Binding of in vitro transcribed leader RNAs to RIG-I.
(A) Anti-Flag M2 immunoprecipitates from cytoplasmic extracts of cells over-expressing Flag RIG-I or Redfp proteins as control (ctrl) were incubated with [32P]-labelled RNA leader or shRNA transcripts made in vitro by the T7 polymerase, in the presence or absence of non hydrolysable ATPγs. Western blotting using Anti-Flag M2 antibody were used to assess the precipitation of Flag-RIG-I on the beads (not shown). (B) A similar experiment was performed with radio-labelled 5′-triphosphate or 5′-monophosphate leader transcribed in vitro by T7 polymerase. Mock (mo): beads incubated with [32P]-radio-labelled RNA in the absence of cytoplasmic extracts. (C) Extracts of cells over-expressing FlagRIG-I (noted wt), FlagRIG-I[K270A] or Redfp as control (ctrl) were incubated with biotinylated 5′-triphosphate measles virus leader RNA, and the complex was then pulled-down with Streptavidin-coupled beads. Proteins were eluted with 500 mM NaCl and analysed by Western blot for RIG-I binding to RNA. Mock represents incubation of RNA without protein. (D) Step gradient elution of FlagRIG-I with either 5′-triphosphate (5′-ppp) or 5′-monophosphate (5′-p) biotinylated measles virus leader RNA. Proteins were sequentially eluted with increasing NaCl concentrations, and analysed for bound RIG-I by western blot.