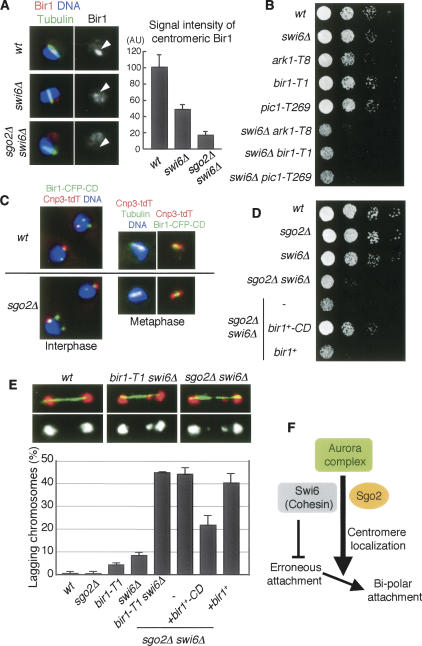
Figure 4.
Forced localization of Bir1 to pericentromeric regions restores sgo2Δ defects. (A) Cells of the indicated strains were stained for Bir1, tubulin, and DNA. Representative images of the indicated cells with short spindles (metaphase) are shown. The signal intensity of Bir1 at metaphase was measured. The error bars represent the SEM (n > 13). (B) Serial dilutions of the indicated strains were spotted onto a YEA plate and incubated at 25°C. (C) Cnp3-tdTomato and Bir1-CFP-CD were observed in wild-type and sgo2Δ cells. (D) Serial dilution of the indicated strains were spotted onto a YEA plate and incubated at 30°C. (E) The indicated strains were cultured at 25°C, fixed, and stained for tubulin and DNA. Examples are shown on top; (red) DNA, (green) tubulin. Frequencies of lagging chromosomes in anaphase cells (n > 100) were examined. The error bars represent the SD of two or three different experiments. (F) A schematic model illustrating how Sgo2 and Swi6 promote bipolar kinetochore–microtubule attachment. A centromeric cohesion defect caused by swi6Δ or cohesin mutation but not by sgo2Δ would provoke erroneous kinetochore–microtubule attachment, which can be restored depending on centromeric Aurora. Since Sgo2 promotes the Aurora localization at centromeres in redundant capacity with Swi6, sgo2Δ swi6Δ double but not either single mutant accumulates erroneous attachment, which is seen as lagging chromosomes.