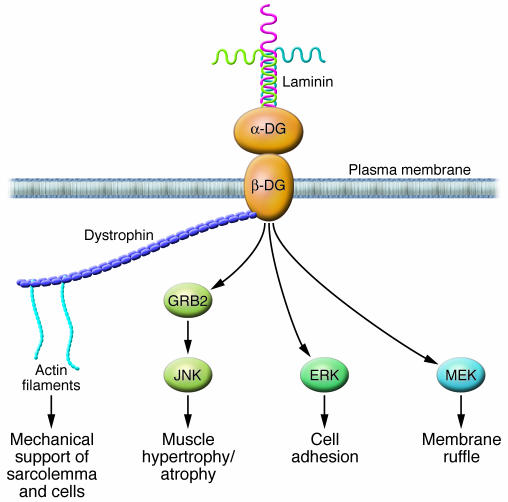
Figure 4. A laminin-dystroglycan-dystrophin signaling cascade.
Dystroglycan (DG) is a key component of the dystrophin-associated glycoprotein complex that provides mechanical support to the sarcolemma. β-Dystroglycan is the transmembrane subunit, and its extracellular domain noncovalently binds α-dystroglycan. Laminin is a major component of the basal laminae and a prominent protein in the endomysium. The binding of laminin to dystroglycan activates a growth factor receptor–bound protein 2–RAC1–PAK1–JNK (GRB2-RAC1-PAK1-JNK) pathway that promotes hypertrophy, an initial adaptive response to increased pressure. Decreased expression of laminin, as might occur during the transition to heart failure, might impair survival signaling to cardiomyocytes and predispose them to apoptosis, similar to the pathology in skeletal muscle dystrophies. Direct association between dystroglycan and MEK and between dystroglycan and ERK was recently demonstrated (82). MEK-dystroglycan association was localized to membrane ruffles, while ERK-dystroglycan association was found in focal adhesions. It is not known how these interactions are regulated.