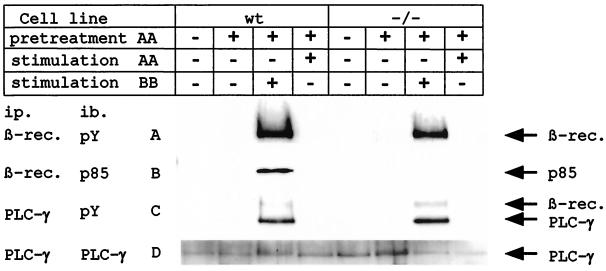
Figure 2.
The mutant PDGF-β receptor is unable to associate with PI3K. Fibroblasts derived from homozygous mutant or wild-type embryos were either completely unstimulated or preincubated with PDGF-AA followed by no further stimulation or stimulation with PDGF-BB or PDGF-AA. Cell lysates derived thereof, containing equal amounts of proteins, were split in two. (A) One-half of the cell lysates was immunoprecipitated with anti-PDGF-β receptor antibodies and blotted with PY20 for the detection of kinase active β receptor. The difference in signal intensity reflects different receptor numbers of the two cell lines (data not shown). (B) The same filter was stripped and reblotted with an anti-PI3K (p85) antibody. (C) The second half of the cell lysates was immunoprecipitated with anti-PLC-γ antibody and blotted with PY20 showing tyrosine-phosphorylated PLC-γ and coimmunoprecipitated, tyrosine-phosphorylated PDGF-β receptor. (D) The same filter was stripped and reblotted with anti-PLC-γ antibody showing similar amounts of PLC-γ. ip, immunoprecipitation; ib, immunoblotting.