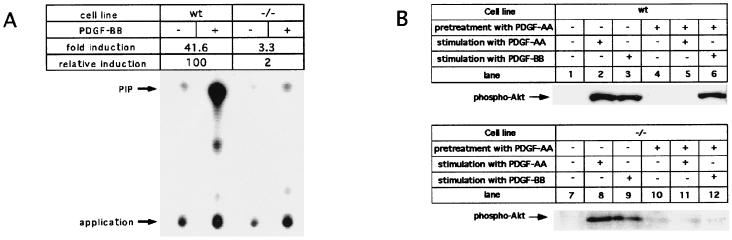
Figure 3.
PI3K activity is not associated with mutant PDGF-β receptor. (A) In vitro PI3K kinase assay. TLC of PI3K reaction products from unstimulated and PDGF-BB-stimulated wild-type and homozygous mutant cells. Cells were incubated without (−) or with (+) 100 ng PDGF-BB/ml at 37°C for 5 min; cell lysates containing identical amounts of total protein (see also Fig. 2) were immunoprecipitated with β receptor-specific antiserum PDGFR3. Immunoprecipitates were subjected to PI3-kinase assay, and PI3K reaction products were analyzed by TLC and autoradiography. The positions of the reaction product, phosphatidylinositol phosphate (PIP), and the application origin are indicated. (B) Phosphorylation of PKB/Akt induced by wt or mutant PDGF receptor. Serum-starved cells were incubated in serum-free starvation medium without ligand (lanes 1 and 7) or in serum-free starvation medium with 25 ng/ml PDGF-AA (lanes 2 and 8) or 25 ng/ml PDGF-BB (lanes 3 and 9) for 5 min; to down-regulate the α receptor, cells were preincubated in serum-free starvation medium for 2 hr with 100 ng/ml PDGF-AA (lanes 4–6 and 10–12), followed by stimulation with 25 ng/ml PDGF-AA (lanes 5 and 11) or 25 ng/ml PDGF-BB (lanes 6 and 12).