Abstract
This review attempts to discuss the role of positron emission tomography (PET) imaging for staging, treatment response and follow-up of patients with lymphoma. The pitfalls and impact of PET imaging on the clinical management are also addressed.
Keywords: Lymphoma, PET imaging, staging, treatment response, follow-up
Introduction
Lymphomas are a group of diseases broadly subdivided into Hodgkin’s (HL) and non-Hodgkin’s lymphomas (NHL) each of which is associated with different presentations, outcomes and therapies. Lymphoma accounts for 5–6% of malignancy in adults in the UK and about 10% of all childhood cancers [1]. NHL is more common than HL by a ratio of approximately 6:1 and has been increasing in incidence over the past 40 years [2, 3]. Males are affected slightly more than females in both types of lymphoma. Hodgkin’s lymphoma is now curable in the majority of patients but NHL has a variable course, ranging from slow and indolent to aggressive and rapidly fatal with a 5 year overall survival of 50–60%.
HL is diagnosed following identification of Reed–Sternberg and Hodgkin cells and shows a bimodal peak distribution occurring in the third decade of life and between 65 and 75 years of age. NHL is a disease mainly of the elderly with an increasing incidence over the age of 50 years and a median age at diagnosis of 65 years [4, 5]. Under the REAL/WHO classifications three categories (i.e. HL, NHL of B cell or T cell/natural killer (NK) cell origin) are recognised [6]. A more in-depth discussion regarding the background to NHL classification can be found elsewhere [1].
HL tends to spread in a contiguous fashion from one lymph node group to the next adjacent group. Primary extranodal HD is very rare. NHL is a disseminated disease involving lymph node groups haphazardly and multiple organs may be involved as well as the bone marrow. Identification of disease in extranodal sites has an adverse effect on prognosis [7]. Whole body imaging is therefore important for accurate staging as this determines management.
The staging system for HL is based on the Cotswold classification [8]. This is of less value in NHL as the prognosis is more dependent on histological grade and other parameters such as tumour bulk and specific organ involvement than on stage [1]. Childhood NHL exhibits a different clinical spectrum with more frequent extranodal involvement primarily involving the gastrointestinal tract, abdominal organs and extranodal sites in the head and neck [9, 10].
Using anatomical imaging lymph node involvement is determined by the presence of nodal enlargement although an increased number of small nodes may be considered suspicious in some clinical circumstances [11]. On the basis of encouraging initial studies positron emission tomography (PET) imaging (using fluorodeoxyglucose (FDG)) has been evaluated extensively in staging, therapy monitoring and surveillance in patients with lymphoma. At primary staging detection of more extensive disease by PET would be of major relevance for patients with apparently limited stage disease. PET may also help to better define radiation treatment volumes in both early and more advanced disease stages by better defining gross tumour volume. Demonstration of disease in normal sized lymph nodes could be of particular importance for planning radiation therapy given that the quality of radiation therapy delivery has a major impact on overall survival [12]. PET-computed tomography (CT) is replacing conventional CT for staging and therapy monitoring except in those where PET is suboptimal (e.g. diabetes). Recent evidence has shown that conventional CT adds little to PET-CT in the evaluation of lymphoma [13].
Staging
The purpose of staging is to define disease extent locally and identify occult disease elsewhere.
Early studies demonstrated that both HL and NHL had an avid uptake of FDG at initial staging [14–16]. In a prospective study involving 60 consecutive patients Moog et al. showed that PET was more accurate for detecting nodal lymphoma than CT. Of 25 additional suspected disease sites found by PET, only two were false positive [14]. Bangerter et al. demonstrated positive PET scans in 38/44 (86%) at sites of documented disease in addition to identifying occult disease sites in five patients (11%). PET identified all 128 abnormal sites identified by conventional imaging plus an additional 11 sites not previously recognised. PET changed management in 14% of cases [15]. In a study of 50 patients no significant difference in sensitivities between PET and CT was demonstrated but PET proved more specific [16]. In another study by Moog comparing PET and CT in 81 patients (43 NHL, 38 HL) 24 additional sites of disease were identified on PET. Most of these sites (93%) were true positive with only one of seven additional findings on CT being positive [17]. A review of 89 consecutive patients comparing CT with PET found that PET had a sensitivity of 98%, a specificity of 94% and an overall accuracy 94% [18]. A subsequent study examining 81 patients with HL demonstrated a staging accuracy for PET of 96% compared with 56% for conventional imaging [19].
A major difficulty is how to accurately validate the results of PET imaging because it is not possible to biopsy all abnormal sites identified. Likewise, it is not possible to biopsy sites that show no abnormality on PET but do so on other imaging. Follow-up may be difficult because the natural history may be altered by treatment and some lymphomas have an indolent nature necessitating a long follow-up period. A prospective study by Young et al. used surgical pathology findings in 11 patients with biopsy of all sites of disease on PET and CT and demonstrated that PET changed stage in 59% of the study population of 45 patients [20].
The majority of studies have determined the accuracy of PET by comparison with CT, other imaging investigations and by clinical follow-up. These studies have grouped together lymphomas of different histological types and patients at various points in the treatment cycle. Most suffer from absence of systematic pathological correlation. Nonetheless, they have demonstrated that PET is at least equal and in many instances superior to other imaging techniques whether used singly or in combination. Although gallium-67 has a well recognised role in lymphoma PET has been demonstrated to be superior in comparative studies [21, 22]. An added advantage of PET is a more convenient imaging protocol coupled with its whole body imaging capability enabling simultaneous visualisation of nodal and extranodal sites.
A problem in staging NHL is the detection of bone marrow infiltration [23]. Magnetic resonance imaging has demonstrated a sensitivity and specificity of approximately 90% in 53 patients (with histological verification) but is a technique that primarily images morphology [24]. A number of studies (with histological verification) indicate that PET is superior to conventional imaging in the evaluation of bone marrow infiltration and may be complementary to bone marrow biopsy. In a study of 50 patients (12 HL, 38 NHL) an overall accuracy of 93% was achieved [25]. A further study comprising 78 patients (39 HL, 39 NHL) found that PET detected bone marrow involvement in 13% of patients but was false negative in 5% [26]. PET was positive in 8/10 discordant cases and led to upstaging in 10%. PET had an overall accuracy of 95% compared with 89% for bone marrow biopsy [26]. However, in a study of 42 patients Jerusalem et al. demonstrated accuracy of detection for PET of only 39% (in biopsy confirmed cases) in indolent NHL [27]. This is likely to be a manifestation of lower FDG uptake by these lymphoma subtypes where the pattern of infiltration may result in difficulty distinguishing it from physiological marrow uptake. In a prospective study comparing bone marrow biopsy with PET and CT in 52 patients PET was significantly more accurate (p<0.05) than CT for all sites and was comparable with bone marrow biopsy for detecting marrow involvement. PET resulted in a change of therapy in 8% of patients [28]. A review by Haioun et al. concluded that PET alone is concordant with conventional imaging and bone marrow biopsy in only 80% of cases, superior to both in 8% and inferior in 12% [29].
Results to date using PET-CT indicate that it is superior to PET or CT alone [13, 30–32]. In the study by Schaefer et al. involving 60 patients (42 HL, 18 high-grade NHL) the sensitivity and specificity for lymph node involvement was 94% and 100% for PET-CT compared with 88% and 86% for contrast enhanced CT [13]. For organ involvement, the sensitivity and specificity was 88% and 100% for PET-CT compared with 50% and 90% for contrast enhanced CT [13]. Although PET-CT performed well for exclusion of disease histological verification was available in only a small number of patients. Other studies with 73 patients and 27 patients demonstrated a significant improvement for PET-CT (p=0.03 and 0.02 respectively) [30, 31]. The study by Hutchings et al. (99 patients) also confirmed the superiority of PET-CT but is the first to demonstrate that caution is required if treatment is to be based exclusively on PET-CT [32]. This study demonstrated upstaging by PET-CT in 10 patients but only disease progression in one (median follow-up 24 months) indicating that more intensive therapy would not have been necessary.
Grading
Studies using semiquantitative measures such as standardised uptake value (SUV) or differential uptake ratio (DUR) have demonstrated that aggressive lymphomas tend to have higher FDG uptake than indolent histologies. Goldberg et al. found that DUR was significantly different for high, intermediate and low-grade NHL (p<0.05) [33]. Okada et al. showed in a study of 34 patients that lymphomas which were aggressive and resistant to treatment tended to show high uptake of FDG and decreased survival [34]. The same author also showed a relationship between a range of quantitative/semiquantitative measures and proliferative activity [35]. High FDG uptake is associated with a high histological grade of malignancy in NHL [36]. In patients with various grades of NHL, FDG can discriminate between high and low grade histologies [37]. Differences in FDG uptake have been demonstrated even within low grade NHL. Jerusalem et al. found that PET identified 40% more abnormal lymph node sites than conventional staging in the 24 patients with follicular histology but <58% of the abnormal CT lymph node sites in the 11 patients with small lymphocytic leukaemia [27]. More recently, Schoder et al. confirmed the findings of earlier studies above and demonstrated that patients with SUV >10 have a high likelihood of aggressive NHL [38]. However, it is worth noting that considerable overlap in SUVs exists in indolent and aggressive NHL in many of the studies with the SUV being determined from the site with the most intense uptake rather than all sites of disease [36–39].
Nonetheless, it is possible to conclude that patients with an SUV 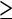 13 at the site of most intense uptake indicates a high probability of aggressive histology while an SUV
13 at the site of most intense uptake indicates a high probability of aggressive histology while an SUV 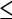 6 is very likely associated with indolent histology [38, 40]. If an SUV cut-off of 10 is used to differentiate between the two groups 29% of aggressive NHL and 19% of indolent NHL would be incorrectly classified resulting in the wrong therapy being administered. However, SUV has an important role in patients where biopsies do not correlate with the clinical findings [40]. If biopsies cannot be easily obtained the possibility of using other markers such as utilisation constant (Ki)
or fluorothymidine (FLT) arises [35, 36, 41–43]. However, Ki is not conducive to being used in routine clinical practice and grading of
lymphoma is of secondary importance to other indications for PET imaging so that FLT only has a limited role [40]. The use of FLT is also limited by its high uptake in bone marrow and liver.
6 is very likely associated with indolent histology [38, 40]. If an SUV cut-off of 10 is used to differentiate between the two groups 29% of aggressive NHL and 19% of indolent NHL would be incorrectly classified resulting in the wrong therapy being administered. However, SUV has an important role in patients where biopsies do not correlate with the clinical findings [40]. If biopsies cannot be easily obtained the possibility of using other markers such as utilisation constant (Ki)
or fluorothymidine (FLT) arises [35, 36, 41–43]. However, Ki is not conducive to being used in routine clinical practice and grading of
lymphoma is of secondary importance to other indications for PET imaging so that FLT only has a limited role [40]. The use of FLT is also limited by its high uptake in bone marrow and liver.
Treatment response
PET is the best non-invasive imaging technique for assessing treatment response [44]. However, FDG is not a perfect indicator of response as it can be influenced by tumour biology, tumour burden at diagnosis, dose and type of chemotherapy regime in addition to the timing of the scan post therapy [44, 45]. Early studies showed that persistence of FDG uptake following treatment was associated with a high relapse rate [46, 47]. Several other studies have confirmed these findings [48–50]. In the largest study comprising 90 patients the probability of complete remission at the end of treatment was 58% if PET remained positive compared with 83% if PET was negative [49]. Analysing the data from 17 end of treatment studies revealed a sensitivity for PET imaging for the detection of residual disease of 76%, specificity 94%, a positive predictive value of 82%, negative predictive value of 92% and an overall accuracy of 89% [44]. Zijlstra et al. performed a metaanalysis of the reported sensitivity and specificity of relevant studies up to 2004 [51]. They reported a pooled sensitivity and specificity for detection of residual disease in Hodgkin’s disease of 84% and 90%, respectively. For NHL, pooled sensitivity and specificity were 72% and 100%, respectively. However, it is important to remember that increased FDG uptake may also arise if active infection or inflammation is present and the PET images should be correlated with clinical findings, other imaging studies and/or biopsy for confirmation before commencing any further therapy [45]. A negative PET scan does not exclude minimal residual disease leading later to a clinical relapse [52].
High dose chemotherapy followed by autologous stem cell transplantation (ASCT) is the treatment of choice for NHL patients relapsing after conventional chemotherapy. It is also the preferred therapy option for most Hodgkin’s disease patients progressing or relapsing after standard chemotherapy. Several studies have shown that FDG-PET during or after reinduction chemotherapy has an important prognostic role in the pretransplantation evaluation of patients with lymphoma [53–57]. In the largest of these studies involving 68 patients the progression free survival was 62% at 2 years for PET negative patients compared with 32% for PET positive patients (p=0.048) [57]. This study also showed that serial PET assessment has a better predictive accuracy than a single PET study. The decision to exclude patients was taken on the basis of PET imaging after stem cell mobilisation which is less than ideal. For patients undergoing allogeneic stem cell transplantation PET has been shown to have a role in monitoring response to adoptive immunotherapy and deciding on further donor lymphocyte infusions [58].
There are five categories in the standardised criteria for response assessment proposed by Cheson and colleagues [11]. The main limitations of CT in the International Workshop Criteria (IWC) are (a) limited accuracy of CT at initial staging for assessing lymphoma in small nodes (<1–1.5 cm), bone marrow or various extranodal sites, (b) inability of CT to identify active disease in a residual mass and (c) limited ability of CT to assess early response to treatment [59]. MRI is particularly useful for assessing the bone marrow and CNS [60]. In a retrospective study of 54 patients with aggressive NHL Juweid et al. showed that a response classification based on integration of FDG-PET with IWC would provide a more accurate response assessment than IWC alone [61]. The greatest discrepancy was present in the CRu group (IWC designation)—patients with no uptake on FDG were designated as CR, those with FDG uptake were designated as PR. All patients reclassified as CR remained progression free at a median of greater than 32 months. In the PR group (IWC) 50% of the patients were reclassified as CR using PET and all but one remained without evidence of disease progression (32 months). Combining IWC and PET provided a statistically significant indicator for progression free survival (p=0.008). The use of PET imaging in this manner is likely to make the CRu category redundant. Likewise, patients with a PR could be redesignated into two subgroups based on PET imaging findings. Validation is required in a prospective trial using a large number of patients following which response is likely to be based exclusively on PET criteria.
Assessing early response to chemotherapy (interim PET imaging)
The desire to instigate an early change in therapy in non-responders arose from a belief that this improved outcome. Standard chemotherapy in NHL patients has been shown to cause a rapid decrease in FDG uptake as early as 7 days after treatment though FDG uptake at 42 days post therapy was superior in prediction of long term outcome [62]. Spaepen et al. evaluated 70 patients with aggressive NHL after 3–4 cycles of therapy and demonstrated that none of 33 patients with abnormal PET imaging achieved a durable complete response whereas 31/37 with a normal PET scan remained in complete response (median follow-up 1107 days) [48]. There was a statistically significant association between PET and progression free survival and overall survival (p<0.00001). PET imaging also achieved a stronger prognostic factor than the international prognostic index. A smaller study
involving 30 patients (17 NHL, 13 HL) showed a statistically significant difference in progression free survival between positive and negative PET patients both after the first cycle and at completion of chemotherapy 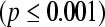 (median follow-up 19 months) [63]. A recent study evaluated 90 patients with aggressive NHL prospectively prior to chemotherapy, at the end of the second cycle and following completion. After completion 83% of patients who were PET negative after two cycles achieved a complete response compared with only 58% of PET positive patients. Outcome also differed significantly with the 2 year estimates of event free survival being 83% compared with 43% (p<0.001)
and an overall survival of 90% compared with 61% (p=0.006) [49]. The predictive value of PET at the end of the second cycle was observed in both the lower and higher risk groups indicating prognostic independence from the international prognostic index. When pooled data from seven studies concerning PET imaging in mid-treatment was analysed the overall sensitivity to predict treatment failure was 79%, specificity 92%, positive predictive value 90% and negative predictive value 81% resulting in an overall
accuracy of 85% [44]. In the largest prospective multicentre evaluation to date PET was able to predict treatment outcome correctly after only two cycles of chemotherapy in 103/108 (95%) patients with Hodgkin’s disease [64]. A further study using 77 patients with Hodgkin’s disease (median follow-up 23 months) showed that a positive PET after two cycles of chemotherapy was associated with reduced progression free survival (p<0.001) and overall
survival (p<0.01) [65]. Early response evaluation using FDG-PET could also help to select patients with a better prognosis thereby allowing a less aggressive
approach with reduced long term toxicity [66]. Further trials are expected to confirm these results indicating the important role of PET imaging in guiding strategy early in the treatment schedule [45].
(median follow-up 19 months) [63]. A recent study evaluated 90 patients with aggressive NHL prospectively prior to chemotherapy, at the end of the second cycle and following completion. After completion 83% of patients who were PET negative after two cycles achieved a complete response compared with only 58% of PET positive patients. Outcome also differed significantly with the 2 year estimates of event free survival being 83% compared with 43% (p<0.001)
and an overall survival of 90% compared with 61% (p=0.006) [49]. The predictive value of PET at the end of the second cycle was observed in both the lower and higher risk groups indicating prognostic independence from the international prognostic index. When pooled data from seven studies concerning PET imaging in mid-treatment was analysed the overall sensitivity to predict treatment failure was 79%, specificity 92%, positive predictive value 90% and negative predictive value 81% resulting in an overall
accuracy of 85% [44]. In the largest prospective multicentre evaluation to date PET was able to predict treatment outcome correctly after only two cycles of chemotherapy in 103/108 (95%) patients with Hodgkin’s disease [64]. A further study using 77 patients with Hodgkin’s disease (median follow-up 23 months) showed that a positive PET after two cycles of chemotherapy was associated with reduced progression free survival (p<0.001) and overall
survival (p<0.01) [65]. Early response evaluation using FDG-PET could also help to select patients with a better prognosis thereby allowing a less aggressive
approach with reduced long term toxicity [66]. Further trials are expected to confirm these results indicating the important role of PET imaging in guiding strategy early in the treatment schedule [45].
Follow-up
Compared to the volume of literature overall regarding PET in lymphoma there is a relative paucity concerning its role in follow-up of the treated patient. A pilot study by Jerusalem et al. involving 36 patients with treated HL underwent PET imaging every 4–6 months for 2–3 years. Identification of active residual or relapsed disease was possible up to 9 months prior to confirmation by conventional imaging or biopsy [67]. This allows early commencement of salvage therapy but a high incidence of false positive results was also recorded (17%). A more recent analysis of data from this group concerning patients with NHL have proved disappointing. As a result they have ceased performing PET imaging as part of routine follow-up in unselected patients with aggressive NHL though they were able to demonstrate a role for PET in detection of preclinical relapse in low grade NHL [45]. Large prospective studies analysing the role and cost benefit of PET in routine follow-up of patients with lymphoma are required. Until then, careful attention to a patient’s history and physical examination with particular regard to those at high risk of relapse remain the best course of action [40].
Pitfalls
It is important to be aware of potential pitfalls in PET imaging of lymphoma patients. Physiological uptake by brown fat and muscles can be diagnosed when compared with CT [68, 69]. PET-CT allows confident identification of tracer within bowel, liver and kidneys. A particular pitfall is to mistake rebound thymic hyperplasia for disease recurrence. However, even uptake within the normal thymus can sometimes lead to confusion. The diffusely increased bone marrow uptake often observed during treatment and related to the administration of growth factor is usually linked to bone marrow hyperplasia and should not be misinterpreted as specific involvement. Comparison with baseline scans may be useful. Treatment related viral and bacterial infections or inflammatory diseases such as sarcoidosis may result in increased FDG uptake sometimes necessitating biopsy [68–71]. False negative results in NHL can occur in mucosa-associated lymphoid tissue (MALT) lymphomas of the gastrointestinal tract and in some low-grade NHLs [72]. This may be related more to organ than nodal uptake of FDG in this condition as others have been able to identify nodal uptake [73]. The requirement or otherwise for i.v. contrast in PET-CT studies is still under discussion. The recent study by Freudenberg et al. using a small number of patients (27) showed good accuracy for PET-CT when compared with PET and conventional post iv contrast CT [31]. Prospective trials with larger number of patients are required before definitive conclusions can be made.
Impact of PET on clinical management of lymphoma
A number of studies have reported change of management that occurred as a result of PET imaging. A change in stage (either upwards or downwards) based on PET does not always result in a change of treatment. In some patients undergoing chemotherapy upstaging from stage III to stage IV may not necessitate a different drug regime whilst in patients undergoing radiation therapy staging may be unaltered but a change in radiation field may be warranted following PET imaging.
In an early paper published in 1996 Valk et al. demonstrated a change in stage in 20% of untreated HL but management change in only 12% [74]. A subsequent paper by Young et al. involving 49 patients with HL demonstrated a change in 59% due to PET imaging though any resulting management changes are not discussed [20]. A study utilising a questionnaire to assess referring physicians’ views indicated that PET changed stage in 44% of patients resulting in intermodality changes of treatment in 42% and intramodality changes of treatment in 10% [75]. Greater than 60% of patients underwent treatment change on the basis of PET imaging. These findings can be contrasted with those of a study involving 50 patients where upstaging occurred in 14% and management change in only 18% [22]. A further paper using a survey of referring physicians found that in 42 patients with childhood lymphoma a change in management occurred in 42% due to PET imaging [76]. Although PET has a well recognised role in aggressive NHL a study by Blum et al. examined its impact in indolent NHL [77]. This study demonstrated that PET changed management in 34% and was best on discordant results (compared with conventional imaging) with an accuracy of 95% (p<0.0001).
Conventional imaging is frequently unable to differentiate between active residual tumour and fibrosis in a residual mass following treatment. In a study of 54 patients PET residual masses were present in 13/19 HL patients and 11/35 NHL patients [46]. A positive PET scan in these patients accurately predicted disease relapse which was supported by the study of Spaepen et al. involving 93 patients [78]. PET scanning in this scenario has an important impact regarding prognosis. Likewise, in patients with stage I or stage II ‘aggressive’ NHL (primarily diffuse large B cell lymphoma) the prognostic information provided by PET could potentially be used to change therapy [79].
As stated earlier although several studies attest to the superiority of PET-CT over CT alone the study by Hutchings et al. (99 patients) is the first to demonstrate that caution is required if treatment is to be based exclusively on PET-CT [13, 30–32]. Further studies examining the impact of PET-CT will be necessary before more definitive conclusions can be reached. For patients undergoing radiation therapy a prospective randomised trial would be necessary to examine the effect on outcome based PET directed radiation therapy. A metaanalysis evaluating the ability of PET to identify bone marrow infiltration in (587 patients) found good (but not excellent) concordance particularly in HL and aggressive NHL [80].
Conclusion
The role of PET imaging in staging, assessing treatment response and in restaging following recurrence means that it is the most common indication in many centres. Continuing improvements in technology coupled with more robust data from prospective studies will enable PET-CT to become the preferred imaging examination in lymphoma with conventional multislice CT and MRI relegated to a secondary role.
References
- 1.Reznek RH, Vinnicombe SJ, Husband JE. 2nd edn. London: Taylor and Francis; 2004. Lymphoma. [Google Scholar]
- 2.Devesa SS, Fears T. Non-Hodgkin’s lymphoma time trends: United States and international data. Cancer Res. 1992;52:5432s–40s. [PubMed] [Google Scholar]
- 3.Cartwright R, Brincker H, Carli PM, et al. The rise in incidence of lymphomas in Europe 1985–1992. Eur J Cancer. 1999;35:627–33. doi: 10.1016/s0959-8049(98)00401-8. [DOI] [PubMed] [Google Scholar]
- 4.Barnes N, Cartwright RA, O’Brien C, Richards ID, Roberts B, Bird CC. Rising incidence of lymphoid malignancies—true or false? Br J Cancer. 1986;53:393–8. doi: 10.1038/bjc.1986.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass AG, Karnell LH, Menck HR. The National Cancer Data Base report on non-Hodgkin’s lymphoma. Cancer. 1997;80:2311–20. [PubMed] [Google Scholar]
- 6.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 7.Shipp MA, Harrington DP, Anderson JR, et al. A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 8.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–6. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 9.Ng YY, Healy JC, Vincent JM, Kingston JE, Armstrong P, Reznek RH. The radiology of non-Hodgkin’s lymphoma in childhood: a review of 80 cases. Clin Radiol. 1994;49:594–600. doi: 10.1016/s0009-9260(05)81874-4. [DOI] [PubMed] [Google Scholar]
- 10.Murphy SB. Childhood non-Hodgkin’s lymphoma. N Engl J Med. 1978;299:1446–8. doi: 10.1056/NEJM197812282992606. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 12.Duhmke E, Diehl V, Loeffler M, et al. Randomized trial with early-stage Hodgkin’s disease testing 30 Gy vs. 40 Gy extended field radiotherapy alone. Int J Radiat Oncol Biol Phys. 1996;36:305–10. doi: 10.1016/s0360-3016(96)00333-1. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer NG, Hany TF, Taverna C, et al. Non-Hodgkin lymphoma and Hodgkin disease: coregistered FDG PET and CT at staging and restaging—do we need contrast-enhanced CT? Radiology. 2004;232:823–9. doi: 10.1148/radiol.2323030985. [DOI] [PubMed] [Google Scholar]
- 14.Moog F, Bangerter M, Diederichs CG, et al. Lymphoma: role of whole-body 2-deoxy-2-[F-18]fluoro-D-glucose (FDG) PET in nodal staging. Radiology. 1997;203:795–800. doi: 10.1148/radiology.203.3.9169707. [DOI] [PubMed] [Google Scholar]
- 15.Bangerter M, Moog F, Buchmann I, et al. Whole-body 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for accurate staging of Hodgkin’s disease. Ann Oncol. 1998;9:1117–22. doi: 10.1023/a:1008486928190. [DOI] [PubMed] [Google Scholar]
- 16.Stumpe KD, Urbinelli M, Steinert HC, Glanzmann C, Buck A, von Schulthess GK. Whole-body positron emission tomography using fluorodeoxyglucose for staging of lymphoma: effectiveness and comparison with computed tomography. Eur J Nucl Med. 1998;25:721–8. doi: 10.1007/s002590050275. [DOI] [PubMed] [Google Scholar]
- 17.Moog F, Bangerter M, Diederichs CG, et al. Extranodal malignant lymphoma: detection with FDG PET versus CT. Radiology. 1998;206:475–81. doi: 10.1148/radiology.206.2.9457202. [DOI] [PubMed] [Google Scholar]
- 18.Bangerter M, Kotzerke J, Griesshammer M, Elsner K, Reske SN, Bergmann L. Positron emission tomography with 18-fluorodeoxyglucose in the staging and follow-up of lymphoma in the chest. Acta Oncol. 1999;38:799–804. doi: 10.1080/028418699432969. [DOI] [PubMed] [Google Scholar]
- 19.Hueltenschmidt B, Sautter-Bihl ML, Lang O, et al. Whole body positron emission tomography in the treatment of Hodgkin disease. Cancer. 2001;91:302–10. [PubMed] [Google Scholar]
- 20.Young CS, Young BL, Smith SM. Staging Hodgkin’s disease with 18-FDG PET. Comparison with CT and surgery. Clin Positron Imaging. 1998;1:161–4. doi: 10.1016/s1095-0397(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 21.Kostakoglu L, Leonard JP, Kuji I, Coleman M, Vallabhajosula S, Goldsmith SJ. Comparison of fluorine-18 fluorodeoxyglucose positron emission tomography and Ga-67 scintigraphy in evaluation of lymphoma. Cancer. 2002;94:879–88. [PubMed] [Google Scholar]
- 22.Wirth A, Seymour JF, Hicks RJ, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography, gallium-67 scintigraphy, and conventional staging for Hodgkin’s disease and non-Hodgkin’s lymphoma. Am J Med. 2002;112:262–8. doi: 10.1016/s0002-9343(01)01117-2. [DOI] [PubMed] [Google Scholar]
- 23.Campbell JK, Matthews JP, Seymour JF, Wolf MM, Juneja SK. Optimum trephine length in the assessment of bone marrow involvement in patients with diffuse large cell lymphoma. Ann Oncol. 2003;14:273–6. doi: 10.1093/annonc/mdg055. [DOI] [PubMed] [Google Scholar]
- 24.Yasumoto M, Nonomura Y, Yoshimura R, et al. MR detection of iliac bone marrow involvement by malignant lymphoma with various MR sequences including diffusion-weighted echo-planar imaging. Skeletal Radiol. 2002;31:263–9. doi: 10.1007/s00256-002-0482-3. [DOI] [PubMed] [Google Scholar]
- 25.Carr R, Barrington SF, Madan B, et al. Detection of lymphoma in bone marrow by whole-body positron emission tomography. Blood. 1998;91:3340–6. [PubMed] [Google Scholar]
- 26.Moog F, Bangerter M, Kotzerke J, Guhlmann A, Frickhofen N, Reske SN. 18-F-fluorodeoxyglucose-positron emission tomography as a new approach to detect lymphomatous bone marrow. J Clin Oncol. 1998;16:603–9. doi: 10.1200/JCO.1998.16.2.603. [DOI] [PubMed] [Google Scholar]
- 27.Jerusalem G, Beguin Y, Najjar F, et al. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) for the staging of low-grade non-Hodgkin’s lymphoma (NHL) Ann Oncol. 2001;12:825–30. doi: 10.1023/a:1011169332265. [DOI] [PubMed] [Google Scholar]
- 28.Buchmann I, Reinhardt M, Elsner K, et al. 2-(Fluorine-18)fluoro-2-deoxy-D-glucose positron emission tomography in the detection and staging of malignant lymphoma. A bicenter trial. Cancer. 2001;91:889–99. [PubMed] [Google Scholar]
- 29.Haioun C, Itti E, Rahmouni A, Meignan M, Reyes F. PET scan in the therapeutic strategy. Hematol J. 2004;5 (Suppl 3):S149–53. doi: 10.1038/sj.thj.6200442. [DOI] [PubMed] [Google Scholar]
- 30.Allen-Auerbach M, Quon A, Weber WA, et al. Comparison between 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography and positron emission tomography/computed tomography hardware fusion for staging of patients with lymphoma. Mol Imaging Biol. 2004;6:411–16. doi: 10.1016/j.mibio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Freudenberg LS, Antoch G, Schutt P, et al. FDG-PET/CT in re-staging of patients with lymphoma. Eur J Nucl Med Mol Imaging. 2004;31:325–9. doi: 10.1007/s00259-003-1375-y. [DOI] [PubMed] [Google Scholar]
- 32.Hutchings M, Loft A, Hansen M, et al. Position emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica. 2006;91:482–9. [PubMed] [Google Scholar]
- 33.Goldberg MA, Lee MJ, Fischman AJ, Mueller PR, Alpert NM, Thrall JH. Fluorodeoxyglucose PET of abdominal and pelvic neoplasms: potential role in oncologic imaging. Radiographics. 1993;13:1047–62. doi: 10.1148/radiographics.13.5.8210589. [DOI] [PubMed] [Google Scholar]
- 34.Okada J, Oonishi H, Yoshikawa K, et al. FDG-PET for predicting the prognosis of malignant lymphoma. Ann Nucl Med. 1994;8:187–91. doi: 10.1007/BF03164996. [DOI] [PubMed] [Google Scholar]
- 35.Okada J, Yoshikawa K, Itami M, et al. Positron emission tomography using fluorine-18-fluorodeoxyglucose in malignant lymphoma: a comparison with proliferative activity. J Nucl Med. 1992;33:325–9. [PubMed] [Google Scholar]
- 36.Lapela M, Leskinen S, Minn HR, et al. Increased glucose metabolism in untreated non-Hodgkin’s lymphoma: a study with positron emission tomography and fluorine-18-fluorodeoxyglucose. Blood. 1995;86:3522–7. [PubMed] [Google Scholar]
- 37.Rodriguez M, Rehn S, Ahlstrom H, Sundstrom C, Glimelius B. Predicting malignancy grade with PET in non-Hodgkin’s lymphoma. J Nucl Med. 1995;36:1790–6. [PubMed] [Google Scholar]
- 38.Schoder H, Noy A, Gonen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:4643–51. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 39.Leskinen-Kallio S, Ruotsalainen U, Nagren K, Teras M, Joensuu H. Uptake of carbon-11-methionine and fluorodeoxyglucose in non-Hodgkin’s lymphoma: a PET study. J Nucl Med. 1991;32:1211–18. [PubMed] [Google Scholar]
- 40.Juweid ME, Cheson BD. Role of positron emission tomography in lymphoma. J Clin Oncol. 2005;23:4577–80. doi: 10.1200/JCO.2005.01.904. [DOI] [PubMed] [Google Scholar]
- 41.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 42.Buchmann I, Neumaier B, Schreckenberger M, Reske S. 18F]3 ′-deoxy-3 ′-fluorothymidine-PET in NHL patients: whole-body biodistribution and imaging of lymphoma manifestations—a pilot study. Cancer Biother Radiopharm. 2004;19:436–42. doi: 10.1089/cbr.2004.19.436. [DOI] [PubMed] [Google Scholar]
- 43.Wagner M, Seitz U, Buck A, et al. 3 ′-[18F]Fluoro-3 ′-deoxythymidine ([18F]-FLT) as positron emission tomography tracer for imaging proliferation in a murine B-cell lymphoma model and in the human disease. Cancer Res. 2003;63:2681–7. [PubMed] [Google Scholar]
- 44.Jerusalem G, Hustinx R, Beguin Y, Fillet G. Evaluation of therapy for lymphoma. Semin Nucl Med. 2005;35:186–96. doi: 10.1053/j.semnuclmed.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Jerusalem G, Beguin Y. The place of positron emission tomography imaging in the management of patients with malignant lymphoma. Haematologica. 2006;91:442–4. [PubMed] [Google Scholar]
- 46.Jerusalem G, Beguin Y, Fassotte MF, et al. Whole-body positron emission tomography using 18F-fluorodeoxyglucose for posttreatment evaluation in Hodgkin’s disease and non-Hodgkin’s lymphoma has higher diagnostic and prognostic value than classical computed tomography scan imaging. Blood. 1999;94:429–33. [PubMed] [Google Scholar]
- 47.Mikhaeel NG, Timothy AR, O’Doherty MJ, Hain S, Maisey MN. 18-FDG-PET as a prognostic indicator in the treatment of aggressive Non-Hodgkin’s lymphoma—comparison with CT. Leuk Lymphoma. 2000;39:543–53. doi: 10.3109/10428190009113384. [DOI] [PubMed] [Google Scholar]
- 48.Spaepen K, Stroobants S, Dupont P, et al. Early restaging positron emission tomography with (18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:1356–63. doi: 10.1093/annonc/mdf256. [DOI] [PubMed] [Google Scholar]
- 49.Haioun C, Itti E, Rahmouni A, et al. 18F]Fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: an early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–81. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 50.Mikhaeel NG, Hutchings M, Fields PA, O’Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–23. doi: 10.1093/annonc/mdi272. [DOI] [PubMed] [Google Scholar]
- 51.Zijlstra JM, Lindauer-van der Werf G, Hoekstra OS, Hooft L, Riphagen II, Huijgens PC. 18F-fluoro-deoxyglucose positron emission tomography for post-treatment evaluation of malignant lymphoma: a systematic review. Haematologica. 2006;91:522–9. [PubMed] [Google Scholar]
- 52.Kazama T, Faria SC, Varavithya V, Phongkitkarun S, Ito H, Macapinlac HA. FDG PET in the evaluation of treatment for lymphoma: clinical usefulness and pitfalls. Radiographics. 2005;25:191–207. doi: 10.1148/rg.251045045. [DOI] [PubMed] [Google Scholar]
- 53.Becherer A, Mitterbauer M, Jaeger U, et al. Positron emission tomography with [18F]2-fluoro-D-2-deoxyglucose (FDG-PET) predicts relapse of malignant lymphoma after high-dose therapy with stem cell transplantation. Leukemia. 2002;16:260–7. doi: 10.1038/sj.leu.2402342. [DOI] [PubMed] [Google Scholar]
- 54.Cremerius U, Fabry U, Wildberger JE, et al. Pre-transplant positron emission tomography (PET) using fluorine-18-fluoro-deoxyglucose (FDG) predicts outcome in patients treated with high-dose chemotherapy and autologous stem cell transplantation for non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2002;30:103–11. doi: 10.1038/sj.bmt.1703607. [DOI] [PubMed] [Google Scholar]
- 55.Filmont JE, Czernin J, Yap C, et al. Value of F-18 fluorodeoxyglucose positron emission tomography for predicting the clinical outcome of patients with aggressive lymphoma prior to and after autologous stem-cell transplantation. Chest. 2003;124:608–13. doi: 10.1378/chest.124.2.608. [DOI] [PubMed] [Google Scholar]
- 56.Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102:53–9. doi: 10.1182/blood-2002-12-3842. [DOI] [PubMed] [Google Scholar]
- 57.Schot B, van Imhoff G, Pruim J, Sluiter W, Vaalburg W, Vellenga E. Predictive value of early 18F-fluoro-deoxyglucose positron emission tomography in chemosensitive relapsed lymphoma. Br J Haematol. 2003;123:282–7. doi: 10.1046/j.1365-2141.2003.04593.x. [DOI] [PubMed] [Google Scholar]
- 58.Hart DP, Avivi I, Thomson KJ, et al. Use of 18F-FDG positron emission tomography following allogeneic transplantation to guide adoptive immunotherapy with donor lymphocyte infusions. Br J Haematol. 2005;128:824–9. doi: 10.1111/j.1365-2141.2005.05388.x. [DOI] [PubMed] [Google Scholar]
- 59.Rahmouni A, Luciani A, Itti E. Quantitative CT analysis for assessing response in lymphoma (Cheson’s criteria) Cancer Imaging. 2005;5(A):S102–6. doi: 10.1102/1470-7330.2005.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahmouni A, Luciani A, Itti E. MRI and PET in monitoring response in lymphoma. Cancer Imaging. 2005;5(A):S106–12. doi: 10.1102/1470-7330.2005.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juweid ME, Wiseman GA, Vose JM, et al. Response assessment of aggressive non-Hodgkin’s lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23:4652–61. doi: 10.1200/JCO.2005.01.891. [DOI] [PubMed] [Google Scholar]
- 62.Romer W, Hanauske AR, Ziegler S, et al. Positron emission tomography in non-Hodgkin’s lymphoma: assessment of chemotherapy with fluorodeoxyglucose. Blood. 1998;91:4464–71. [PubMed] [Google Scholar]
- 63.Kostakoglu L, Coleman M, Leonard JP, Kuji I, Zoe H, Goldsmith SJ. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin’s disease. J Nucl Med. 2002;43:1018–27. [PubMed] [Google Scholar]
- 64.Gallamini A, Rigacci L, Merli F, et al. The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica. 2006;91:475–81. [PubMed] [Google Scholar]
- 65.Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–9. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 66.Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol. 2005;16:1160–8. doi: 10.1093/annonc/mdi200. [DOI] [PubMed] [Google Scholar]
- 67.Jerusalem G, Beguin Y, Fassotte MF, et al. Early detection of relapse by whole-body positron emission tomography in the follow-up of patients with Hodgkin’s disease. Ann Oncol. 2003;14:123–30. doi: 10.1093/annonc/mdg011. [DOI] [PubMed] [Google Scholar]
- 68.Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics. 2004;24:523–43. doi: 10.1148/rg.242025724. [DOI] [PubMed] [Google Scholar]
- 69.Castellucci P, Nanni C, Farsad M, et al. Potential pitfalls of 18F-FDG PET in a large series of patients treated for malignant lymphoma: prevalence and scan interpretation. Nucl Med Commun. 2005;26:689–94. doi: 10.1097/01.mnm.0000171781.11027.bb. [DOI] [PubMed] [Google Scholar]
- 70.Barrington SF, O’Doherty MJ. Limitations of PET for imaging lymphoma. Eur J Nucl Med Mol Imaging. 2003;30 (Suppl 1):S117–27. doi: 10.1007/s00259-003-1169-2. [DOI] [PubMed] [Google Scholar]
- 71.Castellucci P, Zinzani P, Pourdehnad M, et al. 18F-FDG PET in malignant lymphoma: significance of positive findings. Eur J Nucl Med Mol Imaging. 2005;32:749–56. doi: 10.1007/s00259-004-1748-x. [DOI] [PubMed] [Google Scholar]
- 72.Hoffmann M, Kletter K, Diemling M, et al. Positron emission tomography with fluorine-18-2-fluoro-2-deoxy-D-glucose (F18-FDG) does not visualize extranodal B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT)-type. Ann Oncol. 1999;10:1185–9. doi: 10.1023/a:1008312726163. [DOI] [PubMed] [Google Scholar]
- 73.Hicks RJ, MacManus MP, Seymour JF. Initial staging of lymphoma with positron emission tomography and computed tomography. Semin Nucl Med. 2005;35:165–75. doi: 10.1053/j.semnuclmed.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Valk PE, Pounds TR, Tesar RD, Hopkins DM, Haseman MK. Cost-effectiveness of PET imaging in clinical oncology. Nucl Med Biol. 1996;23:737–43. doi: 10.1016/0969-8051(96)00080-7. [DOI] [PubMed] [Google Scholar]
- 75.Schoder H, Meta J, Yap C, et al. Effect of whole-body (18)F-FDG PET imaging on clinical staging and management of patients with malignant lymphoma. J Nucl Med. 2001;42:1139–43. [PubMed] [Google Scholar]
- 76.Montravers F, McNamara D, Landman-Parker J, et al. [(18)F]FDG in childhood lymphoma: clinical utility and impact on management. Eur J Nucl Med Mol Imaging. 2002;29:1155–65. doi: 10.1007/s00259-002-0861-y. [DOI] [PubMed] [Google Scholar]
- 77.Blum RH, Seymour JF, Wirth A, MacManus M, Hicks RJ. Frequent impact of [18F]fluorodeoxyglucose positron emission tomography on the staging and management of patients with indolent non-Hodgkin’s lymphoma. Clin Lymphoma. 2003;4:43–9. doi: 10.3816/clm.2003.n.013. [DOI] [PubMed] [Google Scholar]
- 78.Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of positron emission tomography (PET) with fluorine-18 fluorodeoxyglucose ([18F]FDG) after first-line chemotherapy in non-Hodgkin’s lymphoma: is [18F]FDG-PET a valid alternative to conventional diagnostic methods? J Clin Oncol. 2001;19:414–19. doi: 10.1200/JCO.2001.19.2.414. [DOI] [PubMed] [Google Scholar]
- 79.Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22:3032–8. doi: 10.1200/JCO.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 80.Pakos EE, Fotopoulos AD, Ioannidis JP. 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med. 2005;46:958–63. [PubMed] [Google Scholar]


