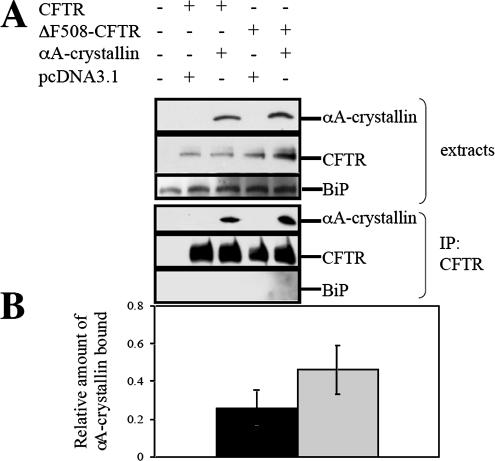
Figure 7.
αA-crystallin coprecipitates preferentially with ΔF508-CFTR. (A) HEK293 cells were transfected with 6 μg of pcDNA3.1-CFTR or pcDNA3.1-ΔF508-CFTR and 2 μg of pcDNA3.1 or pcDNA3. 1-αA-crystallin per 100-mm dish. The interaction between CFTR or ΔF508-CFTR and αA-crystallin was assessed by immunoblot analysis for αA-crystallin after CFTR immunoprecipitation. The ER luminal chaperone BiP served as a negative control. (B) Relative binding efficiency was determined from 3 independent sets of experiments and averaged. All values were obtained after standardization to the amount of CFTR immunoprecipitated. The black bar represents αA-crystallin protein levels coprecipitated with wild-type CFTR, and the gray bar represents αA-crystallin protein levels coprecipitated with ΔF508-CFTR. Vertical bars indicate the SEs of the mean. *p = 0.012 (one-way analysis of variance for 2 correlated samples).