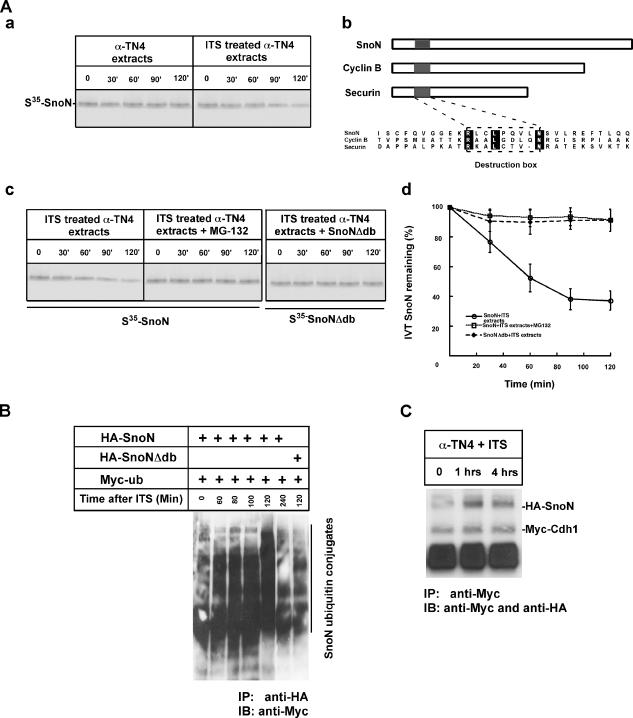
Figure 5.
SnoN is a substrate targeted by Cdh1/APC during lens differentiation. (A) SnoN is degraded in a functional extract prepared from the cells cultured with serum-free ITS induction medium. (a) 35S-labeled in vitro–synthesized SnoN was subjected to extracts prepared from cells without or with serum-free ITS induction. SnoN is stable in uninduced extract, whereas SnoN is degraded in extract prepared from cells induced with serum-free ITS. (b) Alignments of multiple APC substrates including SnoN, cyclin B, and securin. Destruction box, recognition motif in the substrates are indicated. (c) MG132 significantly blocked SnoN degradation. D-box deletion mutant of SnoN failed to be degraded in extracts prepared from cells with serum-free ITS. (d) Quantification of the protein levels for SnoN, p21, and p15 during lens differentiation. (B) Ubiquitylation assay of SnoN during lens differentiation. Ubiquitin tagged with Myc epitope together with SnoN or D-box–deleted SnoN were together transfected into alpha TN4 cells. Ubiquitylation of SnoN was detected by immunoprecipitation of SnoN complex with anti-HA antibody followed by immunoblotting with anti-Myc. As indicated, SnoN is dramatically ubiquitylated after induction although there is a basal ubiquitylation of SnoN before induction. Deletion of the D-box motif significantly decreased SnoN ubiquitylation during lens differentiation. (C) Physical interaction analysis of SnoN and Cdh1 during lens differentiation. Cotransfection of HA-tagged SnoN and Myc-tagged Cdh1 into alpha TN4 cells. Interaction of SnoN and Cdh1 was measured by coimmunoprecipitation at different times as indicated after serum-free ITS induction. SnoN and Cdh1 significantly interacted with each other after 1 h after induction.