Fig. 2.
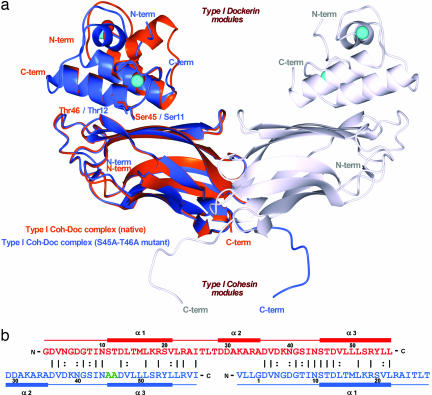
The dual binding mode of the Xyn10B dockerin. (a) Ribbon representation of the superposition of the type I Coh-DocWT complex (in orange) with its S45A-T46A mutant complex (in blue). In the mutant complex, helix-1 (containing Ser-11 and Thr-12) dominates binding whereas, in the WT complex, helix-3 (containing Ser-45 and Thr-46) plays a key role in ligand recognition. Ser-11, Thr-12, Ser-45, and Thr-46, which interact with the cohesin module, are depicted as stick models and colored accordingly. The second molecule of the mutant complex, generated by the 2-fold NCS, is represented in light-gray ribbon. The Ca2+ ions are depicted as spheres and colored orange, in the case of the WT complex, and light blue, in the case of the mutant. The N- and C-terminal ends are labeled and colored accordingly. (b) The structure-based sequence alignment of the WT (in red) and S45A-T46A mutant (in blue) type I dockerins. Mutated residues, Ala-45 and Ala-46, are shown in green. Because of internal 2-fold symmetry of each dockerin module, the two structures overlap almost perfectly in their α1/α3 regions. The N- and C-terminal ends of each module are indicated, as well as the α-helix regions. Numbering is indicated for every 10th residue.