Abstract
The mechanisms leading to degeneration of dopaminergic neurons (DNs) in the substantia nigra of patients with Parkinson's disease (PD) are not completely understood. Here, we show, in the postmortem human tissue, that these neurons aberrantly express mitosis-associated proteins, including the E2F-1 transcription factor, and appear to duplicate their nuclear DNA. We further demonstrate that the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine injected into mice and application of its active metabolite 1-methyl-4-phenylpyridinium to mesencephalic cultures activate the retinoblastoma–E2F pathway in postmitotic DNs. We also find that cell death rather than mitotic division followed the toxin-induced replication of DNA, as determined by BrdU incorporation in DNs. In addition, blocking E2F-1 transcription protected cultured DNs against 1-methyl-4-phenylpyridinium toxicity. Finally, E2F-1-deficient mice were significantly more resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic cell death than their wild-type littermates. Altogether, BrdU incorporation in mature neurons and lack of evidence for newborn neurons argue against neuronal turnover in normal conditions or during pathological states in the substantia nigra. Instead, our results demonstrate that mitosis-like signals are activated in mature DNs in patients with PD and mediate neuronal death in experimental models of the disease. Inhibition of mitosis-like signals may therefore provide strategies for neuroprotection in PD.
Keywords: adult neurogenesis, neurodegeneration, retinoblastoma, apoptosis, dopamine
A progressive degeneration of dopaminergic neurons (DNs) in the substantia nigra pars compacta (SNc) accounts for the debilitating motor symptoms in Parkinson's disease (PD). Although several drugs provide temporary symptomatic benefit, no therapy is currently available to halt disease progression, because the mechanisms that initiate and propagate neuronal cell death are incompletely understood (1).
The present study was inspired by findings that in a number of diverse neurodegenerative diseases mature neurons activate the molecular program that normally guides proliferating cells through the cell cycle to mitotic division, (2–5) and that cell cycle-related signals are implicated in the molecular pathways leading to apoptosis in some neurodegenerative conditions (6–9). There is some evidence that in PD DNs activate the molecular cell-cycle program. Phosphorylation of the retinoblastoma protein (pRb), a molecular trigger of cell-cycle progression, is observed in DNs in the postmortem SNc of PD patients (10). Likewise, cyclin E promotes S-phase entry and is a substrate of the ubiquitin-ligase parkin, mutations of which have been linked to familial PD (11). Other genes that underlie familial forms of PD have also been linked to cancer or implicated in cell-cycle regulation (12). In the 6-hydroxydopamine rat model of PD, cell-cycle markers are expressed in DNs (13). Finally, inhibition of the cell cycle-related kinase cdk2 provides some neuroprotection in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD (14). Together, the available data suggest that cell cycle-associated mechanisms are linked to the process of neurodegeneration in PD. However, there have also been reports suggesting constitutive and lesion-induced neurogenesis in the adult SNc (15). Therefore, the observed cell-cycle events could be related to de novo generation of DNs as well.
Thus, it has not been firmly established whether cell cycle-related signals in DNs in the SNc in PD play a role in cell death processes or are indicative of neurogenesis. In the present study we use human postmortem tissue and in vivo and in vitro models of PD to examine this unresolved issue.
Results
This study consists of a series of experiments defined to explore possible relationships between the molecular pathways of the cell cycle and cell death.
Cell-Cycle Activation in DNs in PD.
We examined DNs in the SNc in postmortem tissue from PD patients for aberrant cell-cycle activation.
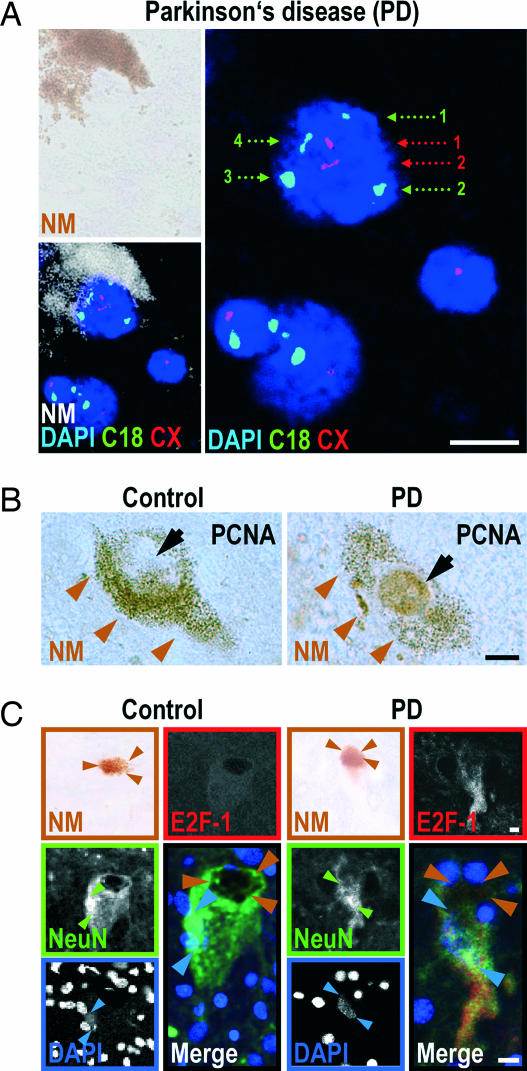
First, we quantified the number of chromosomes in nigral DNs, identified by their neuromelanin content, by in situ hybridization with fluorescent probes for chromosomes X and 18 (4). Observations were made by confocal microscopy. As expected, none of the 232 nuclei analyzed in male control brains contained more than one copy of chromosome X or more than two copies of chromosome 18. In contrast, 4.3 ± 0.8% of the 208 neurons analyzed in male PD patients were polyploid for chromosome 18 and 2.9 ± 0.1% were polyploid for chromosome X [Fig. 1A and supporting information (SI) Table 1].
Fig. 1.
Aberrant cell-cycle activation in DNs of the substantia nigra in PD. (A) DNA duplication in a DN of a male PD patient. Nuclei of DNs were identified by DAPI staining of DNA (blue), which was embedded in a pocket of neuromelanin (NM). In situ hybridization and confocal microscopy allowed quantification of the number of chromosomes X (CX, red hybridization spots, red numbering) and 18 (C18, green hybridization spots, green numbering). The dopaminergic NM+ neuron in Upper Right contains four copies of chromosome 18 and two copies of chromosome X, indicating that the chromosomes in this cell had been duplicated. The two small nondopaminergic NM-negative nuclei in Lower Right contain two chromosomes 18 and one chromosome X, as expected for a cell not undergoing mitosis. (B) In PD patients, but not in controls, pronounced nuclear PCNA immunoreactivity (brown diaminobenzidine precipitate) was observed (black arrows) in DNs, identified by their cytoplasmic NM content (brown arrowheads). (C) In PD patients, but not in controls, pronounced cytoplasmic E2F-1 immunoreactivity (red) was observed in DNs, identified by their NM content (brown; brown arrowheads). NeuN immunostaining (green) delineated the cell bodies (weak staining) and nuclei (intense staining; green arrowheads). DAPI confirmed the location of the nuclei (blue; blue arrowheads). The fluorescent images are confocal. The merged image superposes E2F-1 (red), NeuN (green), and DAPI (blue) staining. (Scale bars: 10 μm.)
Second, immunoreactivity for proliferating cell nuclear antigen (PCNA), a subunit of the DNA polymerase (16), was also found in the nucleus of 5.9 ± 1.9% of the 2,593 neurons analyzed in PD patients (Fig. 1B). Under identical staining conditions, no such immunoreactivity was observed in 11,634 neurons analyzed in controls.
Third, immunoreactivity for E2F-1, a mitosis-linked transcription factor (16), was found predominantly in the cytoplasm of 3.5 ± 0.9% of the 1,569 neurons analyzed in PD patients (Fig. 1C). Under identical staining conditions, no such staining was observed in 1,482 neurons analyzed in controls.
These observations provide strong evidence that aberrant cell-cycle processes are activated in DNs in PD.
PCNA Expression in DNs in MPTP-Treated Mice.
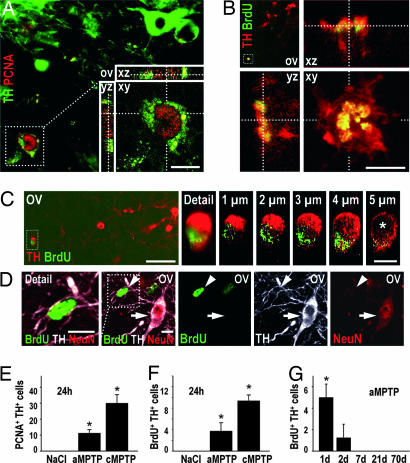
To determine whether neurons also initiate cell-cycle processes in a model of PD, we specifically killed DNs in the SNc of mice treated with MPTP (1), in acute (4 × 20 mg/kg i.p. at 2-h intervals) or subchronic (5 × 30 mg/kg at 24-h intervals) paradigms, and killed the mice 24 h later. PCNA+ nuclei were observed in nigral DNs, identified by tyrosine hydroxylase (TH) expression, only in MPTP-treated mice, but not in the controls (Fig. 2 A and E).
Fig. 2.
Nuclear DNA synthesis in DNs in the SNc of mice 24 h after MPTP intoxication. (A) Nuclear PCNA immunoreactivity (red) in a DN, identified by TH expression (green) is shown. An overview (ov) and an inset showing a higher magnification of the cell in a confocal reconstruction of the xy, xz, and yz planes are shown. (B) BrdU immunoreactivity (green) in a TH+ neuron (red). Overview (ov) and detail of the boxed area showing the cell in a confocal reconstruction of the xy, xz, and yz planes. The superposition of BrdU and TH appears yellow, demonstrating that both markers colocalize in the same cellular compartment, suggesting a breakdown of the nuclear membrane. Note the disintegrated TH+ cytoplasm and the condensed BrdU+ chromatin, suggesting that the cell is in a state of degeneration. (C and D) Examples of artifactual BrdU-TH colocalization. (C) A BrdU+ nucleus (green) that erroneously appears to belong to a TH+ neuron (red) when seen with conventional microscopy: an overview (ov) and an enlarged detail in the boxed area are shown. However, confocal microscopy of sections 1 μm apart shows that the BrdU+ nucleus is juxtaposed to the TH+ cell and that the nucleus of the TH+ cell (best seen at 5 μm; ∗) is not BrdU+. (D) A BrdU+ nucleus (green) that erroneously appears to belong to a TH+ neuron (white), when observed by confocal microscopy. An overview (ov) and an enlarged detail are shown). However, the lack of counterstaining with the neuronal marker NeuN (red) demonstrates that the BrdU+ nucleus (arrowhead) is not neuronal in nature, in contrast to the neighboring TH+ BrdU− cell (arrow). The TH+ immunoreactivity in the vicinity of the BrdU+ nucleus is contained in the neurites of neighboring DNs. (Scale bars: 10 μm.) (E and F) Absolute numbers of PCNA+ TH+ cells (E) and BrdU+ TH+ cells (F) per SNc of mice 24 h after NaCl injection (control) or after intoxication with MPTP in the acute (aMPTP) or subchronic (cMPTP) paradigm. ∗, P < 0.05, ANOVA followed by post hoc test, vs. NaCl. (G) Absolute numbers of BrdU+ TH+ cells per SNc of mice at different time points (d = days) after MPTP intoxication in the acute paradigm (aMPTP). Two-way ANOVA showed a significant effect of treatment (MPTP vs. NaCl, P < 0.001), time (P < 0.01), and treatment × time interaction (P < 0.01). ∗, P < 0.05, post hoc test, vs. NaCl.
DNA Synthesis in DNs in MPTP-Treated Mice.
To determine whether DNA is synthesized in DNs of MPTP mice, we injected the thymidine analogue BrdU immediately after the first MPTP injection (acute MPTP model: 5 × 50 mg BrdU/kg i.p. at 6-h intervals; subchronic MPTP model: 10 × 50 mg/kg at 12-h intervals; n = 4 per group). BrdU was incorporated into nuclear DNA of TH+ cells in both the acute and the subchronic MPTP model, but not in NaCl/BrdU-injected controls (Fig. 2 B and F).
BrdU+ TH+ Cells Appear Early and Disappear Rapidly After MPTP Intoxication.
To analyze the kinetics of the appearance of BrdU+ TH+ cells, we treated mice with MPTP and BrdU (acute paradigm) and killed them after 1, 2, 7, 21, or 70 days (n = 3 per time point). In many instances, apparent colocalization of BrdU with TH was artifactual, as evidenced by confocal microscopy or colabeling with the neuronal marker NeuN (Fig. 2 C and D). In NaCl/BrdU-injected control mice (n = 3 per time point), not a single unequivocal BrdU+ TH+ cell was observed during this time course. Convincing double-stained BrdU+ TH+ cells were seen only on days 1 and 2 after MPTP intoxication (Fig. 2G).
No Evidence for Persistent Neurogenesis in the SNc.
Because there was a previous report of continuous production of BrdU+ TH+ cells in mice (17), we acutely injected mice with MPTP or NaCl, followed by BrdU injection, according to the paradigms described in SI Table 2, and killed them 70 days later. No BrdU+ TH+ cells were found in the SNc of these mice, arguing against the existence of spontaneous or lesion-induced generation of new TH+ neurons that would persist for 70 days.
No Evidence for Transient Neurogenesis in the SNc.
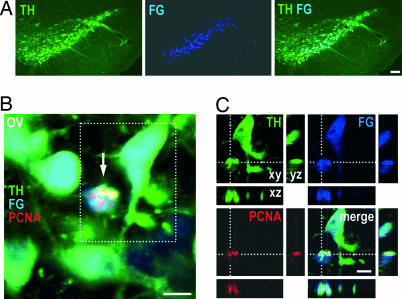
To test the possibility that the PCNA+ TH+ cells observed soon after MPTP intoxication were newborn neurons that failed to survive, we injected the retrograde tracer FluoroGold into the striatum, the major projection target of the SNc, labeling >90% of the nigral TH+ neurons (Fig. 3A). These mice were then acutely or subchronically treated with MPTP or NaCl (n = 4 per group) and analyzed 24 h later. All identified PCNA+ TH+ cells were also FluoroGold-positive (Fig. 3 B and C), demonstrating that they already existed before intoxication and were thus not newborn cells.
Fig. 3.
PCNA-expressing dopaminergic cells in the SNc of MPTP mice are not newborn neurons, but preexist. (A) TH+ neurons (green) in the SNc were labeled by striatal injection of the retrograde tracer FluoroGold (FG, blue) 3 days before MPTP intoxication. (B) Overview (ov) showing a cell (arrow) in the SNc of a mouse 24 h after acute MPTP intoxication expressing both TH (green) and PCNA (red). The presence of FluoroGold (blue) demonstrated that the cell existed before the lesion, ruling out the possibility that it was a stem cell-derived newborn dopaminergic cell. (C) An enlarged detail from the boxed area in B shown as a confocal reconstruction in the xy, xz, and yz plane, to confirm the colocalization of TH, PCNA, and FG. [Scale bars: 100 μm (A); 10 μm (B and C).]
G1–M-Phase Markers in Degenerating Neurons.
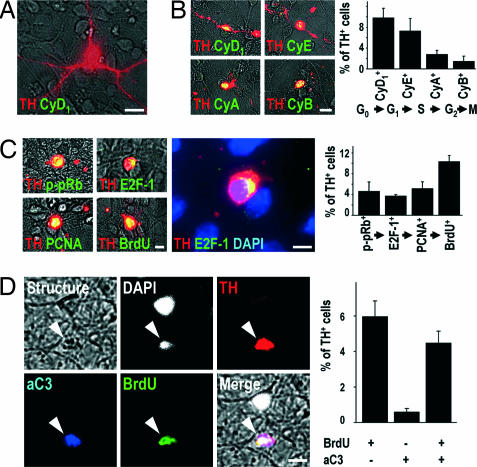
To study DNs in a more efficient manner (18), we prepared primary cultures of embryonic rat midbrain neurons that were terminally postmitotic under control conditions, because they did not stain for the cell cycle-related molecules described below (Fig. 4A). However, after 24 h of exposure to 1-methyl-4-phenylpyridinium (MPP+), the toxic metabolite of MPTP, TH+ neurons became strongly immunoreactive for the G1-phase-associated cyclin D1, S-phase-associated cyclin E, G2-phase-associated cyclin A, and M-phase-associated Cyclin B in decreasing frequency (Fig. 4B). Cyclin D1+ neurons still had rather well preserved dendrites, whereas those expressing the late-phase-associated cyclin B had completely lost their arborization (Fig. 4B). This finding suggests that MPP+-treated TH+ neurons activate signals from each phase of the cell cycle as they degenerate. The fact that fewer cells were cyclin B+ than cyclin D1+ may signify that some cells die before reaching an M-phase-like state.
Fig. 4.
Cell-cycle events do not occur in postmitotic TH+ midbrain neurons in vitro under control conditions (A), but are observed after MPP+ intoxication (B–D). (A) In control cultures, cell cycle-associated proteins, such as cyclin D1 (CyD1, green) were never expressed in TH+ neurons (red). (B) However, after 24 h of intoxication with MPP+, the G1-phase-associated CyD1, the S-phase-associated cyclin E (CyE), the G2-phase-associated cyclin A (CyA), and the M-phase-associated cyclin B (CyB) (green) were expressed in TH+ cells (red). The graph shows the percentage of TH+ cells expressing CyD1, CyE, CyA, and CyB after 24 h of MPP+ intoxication. (C) Immunoreactivity for the S-phase-associated markers p-pRb, E2F-1, PCNA, and BrdU (green) was detected in TH+ DNs (red) after 24 h of MPP+ intoxication. Visualization of chromatin with DAPI (blue) demonstrated the perinuclear expression of E2F-1 (green) in TH+ neurons (red). The graph shows the percentage of TH+ cells immunoreactive for p-pRb, E2F-1, PCNA, and BrdU after 24 h of MPP+ intoxication. (D) A 24-h treatment of cultured neurons with MPP+ led to the appearance of morphologically disintegrated cells (structure, gray) with condensed chromatin (DAPI, white) and dopaminergic phenotype (TH+, red) that were immunopositive for both the activated form of the downstream effector caspase-3 (aC3, blue) and BrdU (green). The graph shows the quantification of the presence (+) or absence (−) of BrdU and aC3 immunoreactivity in TH+ cells after 24 h of MPP+ treatment. [Scale bars: 20 μm (A and B); 10 μm (C and D).]
G1/S-Phase Transition in Degenerating Neurons.
As a next step, we sought out to determine whether cells progressed toward S phase in the expected manner. The S phase is typically initiated by phosphorylation of pRb, leading to the release of E2F transcription factors. Unbound E2F proteins autoinduce E2F gene expression and transactivate E2F-target genes, such as PCNA, which is required for DNA synthesis (16). After MPP+ intoxication, but not in control conditions, numerous TH+ cells were immunoreactive for phosphorylated pRb (p-pRb), E2F-1, PCNA, or BrdU (Fig. 4C), suggesting that DNA replication in degenerating TH+ neurons follows the expected program. Fewer cells were positive for p-pRb, E2F-1, or PCNA+ than for BrdU, however. This discrepancy might be caused by the lower probability of detecting transiently activated signals in cells passing through the G1/S transition than BrdU that becomes permanently incorporated.
Co-Occurrence of BrdU Incorporation and Caspase-3 Activation.
To test whether the cell cycle and cell death were activated simultaneously or mutually exclusively, we stained for BrdU and caspase-3. Among the 643 MPP+-intoxicated TH+ neurons analyzed in vitro, 88% of those containing activated caspase-3 also had BrdU+ nuclei, suggesting that the programs for cell cycle and apoptosis are activated concomitantly (Fig. 4D).
Cell-Cycle Signals in Apoptotic TH+ Cells in the SNc.
We then asked whether the cell-cycle signals that we observed in vitro were recapitulated in vivo. TH+ apoptotic cells, identified according to morphological criteria (13) 24 h after acute MPTP intoxication in vivo, were p-pRb+ and E2F-1+, suggesting that, like in vitro, degenerating neurons activate cell-cycle signals in vivo (SI Fig. 6).
Caspase Inhibition Does Not Prevent BrdU Incorporation.
To understand the hierarchy of cell cycle/cell death signaling, we inhibited caspase signaling. In MPP+-intoxicated TH+ neurons in vitro, inhibition of caspases with the broad-spectrum inhibitor zVAD-fmk did not prevent BrdU incorporation (Fig. 5 A and B), suggesting that caspase activation is not functionally upstream of DNA synthesis.
Fig. 5.
E2F-1 signaling is upstream of MPP+-induced caspase-3 activation, and E2F-1 deficiency provides protection against MPTP. (A and B) Caspase activation is not upstream of cell-cycle signaling. Caspase inhibition (CI) with 200 μM zVAD-fmk during a 24-h intoxication of TH+ neurons (red) with MPP+ prevented the appearance of immunoreactivity for the activated form of caspase-3 (aC3, green) (A), but not the incorporation of BrdU (green) (B). DAPI (blue) stains nuclei. The superposition of red and green appears yellow. The superposition of red, green, and blue appears white. The graphs show the percentage of TH+ cells immunoreactive for aC3 (A) and BrdU (B). ∗, P < 0.05; n.s., not significant. (C–E) E2F-1 inhibition protects neurons in vitro from MPP+. Inhibition of E2F-1 expression with antisense (AS), but not sense (S) oligonucleotides during a 24-h intoxication of TH+ DNs (red) with MPP+ attenuated the appearance of immunoreactivity for E2F-1 (green) (C) and aC3 (green) (D) and the MPP+-induced loss of TH+ cell (E). DAPI (blue) stains nuclei. The graphs show the percentage of TH+ cells immunoreactive for E2F-1 (C) and aC3 (D) and the percentage of surviving TH+ cells (E) after transfection with E2F-1 sense (S) or antisense (AS) oligonucleotides and grown for 24 h in the absence (C) or the presence (M) of MPP+. ∗, P < 0.05; ∗∗∗, P < 0.001; n.s., not significant. (F–I) E2F-1 deficiency protects neurons in vivo from MPTP. (F) Dopaminergic (TH+) cell bodies in the SNc, assessed 7 days after MPTP intoxication in both the subchronic (cMPTP) and acute paradigm (aMPTP), were significantly protected in E2F-1−/− mice compared with E2F-1+/+ mice. ∗, P < 0.05; n.s., not significant. (G) Representative photomicrographs of TH+ cell bodies in the SNc in E2F-1−/− mice compared with E2F-1+/+ mice 7 days after MPTP intoxication. (H) The striatal dopamine depletion, assessed 7 days after MPTP intoxication in both paradigms did not differ between E2F-1−/− and E2F-1+/+ mice. (I) The time course of the accumulation and elimination of the toxic MPTP-metabolite MPP+ after an injection of MPTP was identical in E2F-1−/− mice and wild-type littermates (E2F-1+/+). [Scale bars: 10 μm (A-D); 100 μm (E and G).]
E2F-1 Suppression Prevents MPP+-Induced Caspase-3 Activation.
We then tested, conversely, whether cell-cycle activation is upstream of caspase activation in the MPP+ model in vitro. E2F induction is a bottleneck event upstream of PCNA expression and DNA replication (14). E2F-1 and E2F-4 are the most abundant E2F family members in the brain, but E2F-1, unlike E2F-4, is predominately regulated by p-pRb. Furthermore, E2F-1 has been shown to be the E2F family member that most potently sensitizes cells for apoptosis (16, 19). Thus, we chose to inhibit E2F-1 expression by transfection of cultured neurons with a validated E2F-1 antisense oligonucleotide; transfection efficiency was 80% (20). E2F-1 sense oligonucleotides, missense oligonucleotides (data not shown), and omission of oligonucleotides (data not shown) were used as controls. The antisense oligonucleotide, but not the controls, decreased E2F-1 expression, activation of caspase-3, and loss of TH+ cells in MPP+-intoxicated cultures (Fig. 5 C–E), suggesting that E2F-1 signaling is upstream of caspase activation and cell death in this model.
E2F-1−/− Mice Are Protected Against MPTP.
Finally, we tested whether E2F-1-deficiency in mice (19) could mitigate MPTP toxicity in vivo. Indeed, after both acute and subchronic MPTP intoxication, stereological cell counts demonstrated that there was significantly less nigral TH+ cell loss in E2F-1−/− mice than in E2F-1+/+ mice (Fig. 5 F and G). Yet, the extent of striatal dopamine depletion was in the same range in both genotypes (Fig. 5H). Finally, the striatal accumulation and elimination of the toxic metabolite MPP+ after a single injection of 30 mg/kg MPTP was similar in E2F-1−/− and E2F-1+/+ mice (Fig. 5I), suggesting that decreased MPTP metabolism did not account for neuroprotection afforded by E2F-1 deficiency.
Discussion
Although neurons of the mammalian central nervous system are terminally differentiated and unable to divide, the present study shows that cell cycle-related processes are active in the DNs of the SNc in patients with PD and that these processes are upstream events in the molecular pathway leading to neuronal cell death in experimental models of the disease. The present study has widespread clinical and conceptual significance for research on cell death in PD and on adult neurogenesis as a whole.
Consistent with a recent study showing aberrant pRb phosphorylation in DNs in the postmortem SNc of PD patients (10), we found p-pRb in TH+ neurons in MPTP-treated mice and MPP+-intoxicated cultures. Moreover, we found aberrant expression of E2F-1 and the E2F-inducible protein PCNA in DNs of PD patients, MPTP-treated mice, and MPP+-treated cultures. Although a previous study came to a different conclusion (14), the present observations provide strong evidence of aberrant activation of the pRb/E2F pathway, a core molecular pathway leading to initiation of the mitotic S phase (7), in DNs in PD and the MPTP/MPP+ model.
The mechanisms activating the pRb/E2F pathway in PD and models of the disease are unknown. Yet, oxidative stress and dysfunction of the proteasomal protein degradation pathway, which have been implicated in the pathophysiology of PD (18), can trigger cell-cycle activation in postmitotic neurons (21, 22) and are thus candidate mechanisms for future studies into this field.
We have demonstrated by FISH that nuclear DNA is duplicated in nigral DNs in PD patients. In both MPTP-treated mice and the MPP+-treated cell cultures, we observed nuclear DNA synthesis in DNs as shown by incorporation of the thymidine analogue BrdU. Although BrdU incorporation may also result from DNA repair, the concomitant expression of cell cycle-related proteins suggests that it was most likely caused by DNA duplication. Our observations together with other studies (10, 13) therefore suggest that the cell cycle-related signals observed in PD patients and models of the disease are functionally relevant and lead effectively to DNA synthesis.
Incorporation of BrdU into DNs has already been observed in the mouse SNc after MPTP intoxication and was interpreted as a sign of adult neurogenesis (17). However, our FluoroGold tracing experiments unequivocally demonstrate that the cell-cycle-like processes observed in vivo occurred in neurons that were already present before their intoxication. Thus, in agreement with other studies (15, 23, 24), we found no evidence for spontaneous or lesion-induced, transient or persistent, generation of new TH+ neurons in the adult SNc.
Several of our observations suggest that cell cycle-like processes are activated in neurons during degeneration: the condensed appearance of the BrdU+ chromatin and the disintegrated cytoplasm of the BrdU+ cells in MPTP mice (Fig. 3B); and the progressive morphological disintegration of MPP+-exposed cultured neurons during the period in which they expressed cyclins that regulate later stages of the cell cycle (Fig. 4B). The observation that nearly all DNs with activated caspase-3 had also incorporated BrdU into their DNA clearly demonstrated that there is a close association between cell cycle-like processes and apoptosis in the MPTP/MPP+ model of PD, as was suggested in the 6-hydroxydopamine model of PD on the basis of morphological criteria (13).
Mitosis and apoptosis are highly conserved mechanisms by which eukaryotic cells divide or die, respectively. Recent evidence suggests that these processes share molecular mediators, such as pRb and the E2F family of transcription factors (6–9). The transcriptional activity of E2F prepares cells for DNA synthesis during mitosis (16). Simultaneously, E2F activity sensitizes cells to apoptosis by inducing transcription of proapoptotic proteins (16, 25). This Janus-faced activity provides cycling cells with the ability to escape inappropriate proliferative and potentially oncogenic signals by inducing apoptosis.
Consistently, we observed that the cell cycle-like events in cultured TH+ neurons are not a consequence of apoptosis-associated caspase activity. Rather, E2F-1 seems to acts upstream of caspase-3 activation and neuronal cell death. It is noteworthy that the neuroprotective effect of E2F-1 inhibition was observed in pure neuronal cultures, suggesting that it resulted from the blockade of cell death signaling cascades in the DNs themselves rather than from repression of a putative glia-dependent mechanism. Consistent with the in vitro data, mice deficient in E2F-1 were also significantly protected against loss of nigral TH+ neurons after MPTP intoxication. E2F-1 was originally proposed to be the only member of the E2F family that induces apoptosis, although recent data suggest that other E2F proteins trigger apoptosis as well (16). Nonetheless, in the MPTP/MPP+ model of PD, E2F-1 appears to be a crucial upstream mediator of dopaminergic neurodegeneration. Because neuronal cell death was not completely blocked in the absence of E2F-1, compensatory activities of other E2F family members will need further investigation.
Unlike neuronal death in the MPTP/MPP+ model, neuronal loss in the SNc in PD patients proceeds slowly over several decades. One would, therefore, expect to observe far fewer dying cells at any given moment than the 4.3% found to be aneuploid for chromosome 18. Thus, there appears to be a temporal dissociation between DNA replication and cell death in PD, suggesting that the aneuploid neurons might be arrested in a G2-like state for a prolonged period before their demise (5), for reasons that are presently unknown.
In conclusion, our data do not only show that the pRb/E2F pathway is activated in DNs in PD, but also demonstrate that activation of this pathway is instrumental in the degeneration of these neurons in the MPTP/MPP+ model of the disease, as summarized in SI Fig. 7. These findings suggest that cell-cycle regulators may be effective targets for therapeutic strategies aimed at slowing or halting neurodegeneration in PD.
Materials and Methods
Human Brains.
Autopsy tissue containing the SNc from pathologically confirmed PD patients and individuals without neurological disorders was obtained in accordance with local law from the Institut National de la Santé et de la Recherche Médicale Unité Mixte de Recherche 679 Brain Bank and the Harvard Brain Tissue Resource Center (Cambridge, MA). Details are published in SI Text.
Animals.
The appropriate animal care committees approved the work. Wild-type mice were C57BL/6 (Janvier Breeding Center, LeGenest St. Isle, France). E2F1−/− mice (189) were backcrossed 12 times onto the C57BL/6 background. E2F1−/− and E2F1+/+ littermates were obtained by crossing E2F1+/−. Genotyping was done by PCR (19).
MPTP.
Ten-week-old male mice received MPTP HCl (Sigma–Aldrich, Lyon, France) or equal volumes of 0.9% NaCl (controls) as described (1).
BrdU.
BrdU (Sigma) was dissolved at 5 mg/ml in 0.9% NaCl and injected i.p at doses of 50 mg/kg body weight.
FluoroGold.
FluoroGold (0.4 μl 4%) was injected in the striatum (coordinates: A +1.2, L ±1.6, V −2.5 mm) of anesthetized mice (10 ml/kg of 1% ketamine/0.2% xylazine).
Tissue Preparation.
For immunohistochemistry, mice were killed with 100 mg/kg pentobarbital i.p. and perfused transcardially. Postfixed frozen brains were cut in 20-μm coronal sections. For HPLC, mice were killed by cervical dislocation. The dissected striata were homogenized in 500 μl of 0.4 M perchloric acid, centrifuged (20 min, 13,000 × g, 4°C), and passed through a 0.2-μm filter.
HPLC.
Dopamine was measured by RP-HPLC with electrochemical detection (potential 750 mV) under isocratic conditions with an Ag/AgCl reference electrode. MPP+ was determined by HPLC with UV detection (wavelength 295 nm).
Cell Culture, Immunohistochemistry, and Image Analysis.
Details are published in SI Text.
Antisense Oligonucleotides.
Thirty minutes before intoxication, mouse cultures were transfected with 0.35 μM of mouse-specific E2F-1 antisense (5′-GAA GCG TTT GGT GGT CAG AT-3′), E2F-1 sense (5′-ATC TGA CCA CCA AAC GCT TC-3′), or missense (5′-TTG CCT CCC TTT GAA AAA TG-3′) oligonucleotides or without oligonucleotides as described (20).
In Situ Hybridization.
Ten-micrometer cryostat sections of human SNc were fixed at −20°C in 3:1 methanol/acetic acid, rinsed in a graded alcohol series, digested with 0.5% pepsin in 0.01 M HCl (13 min, 37°C), washed in PBS, postfixed in 1% paraformaldehyde (5 min), washed, and dehydrated in a graded alcohol series. Nuclei and DNA probes (CEP; Vysis, Des Plaines, IL) were denatured (76°C, 2 min) and hybridized overnight in a humidified chamber (37°C). Sections were rinsed in 0.4× saline-sodium citrate with 0.3% Nonidet P-40 (American Bioanalytical, Natick, MA) (72°C, 2 min) and 2× SSC with 0.1% Nonidet P-40 (20°C, 5 min). Autofluorescence was reduced with Sudan black. Vysis control slides and female tissue (for chromosome X probes) were hybridized to ensure sensitivity and specificity.
Statistics.
Data are shown as mean ± SEM. Normal parametric data were compared with the two-sided, unpaired t test or ANOVA followed by post hoc Student-Newmann-Keuls test. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank S. Stei for technical assistance and the Harvard Brain Tissue Resource Center for postmortem tissue. This work was supported by Harvard Brain Tissue Resource Center Grant R24 MH 68855, German Ministry of Education and Research Grants BMBF-01GN0513 and 01GO0201, European Union Grant LSHM-CT-2003-503330, the Peter Hofmann Project, Fondation pour la Recherche Médicale Grant ACE20030307094, the Institut National de la Santé et de la Recherche Médicale, and the Public Health Service (P.R.).
Abbreviations
- DN
dopaminergic neuron
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PCNA
proliferating cell nuclear antigen
- PD
Parkinson's disease
- pRb
retinoblastoma protein
- p-pRb
phosphorylated pRb
- SNc
substantia nigra pars compacta
- TH
tyrosine hydroxylase.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611671104/DC1.
References
- 1.Dauer W, Przedborski S. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Jordan-Sciutto KL, Wang G, Murphey-Corb M, Wiley CA. J Neurosci. 2002;22:2185–2195. doi: 10.1523/JNEUROSCI.22-06-02185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranganathan S, Bowser R. Am J Pathol. 2003;162:823–835. doi: 10.1016/S0002-9440(10)63879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Geldmacher DS, Herrup K. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Mufson EJ, Herrup K. J Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen MD, Mushynski WE, Julien JP. Cell Death Differ. 2002;9:1294–1306. doi: 10.1038/sj.cdd.4401108. [DOI] [PubMed] [Google Scholar]
- 7.Greene LA, Biswas SC, Liu DX. Cell Death Differ. 2004;11:49–60. doi: 10.1038/sj.cdd.4401341. [DOI] [PubMed] [Google Scholar]
- 8.Herrup K, Neve R, Ackerman SL, Copani A. J Neurosci. 2004;24:9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuan CY, Schloemer AJ, Lu A, Burns KA, Wenig W-L, Williams M-T, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, et al. J Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan-Sciutto KL, Dorsey R, Chalovich EM, Hammond RR, Achim CL. J Neuropat Exp Neurol. 2003;62:68–74. doi: 10.1093/jnen/62.1.68. [DOI] [PubMed] [Google Scholar]
- 11.Staropoli JF, McDermott C, Martinat C, Schulman B, Demireva E, Abeliovich A. Neuron. 2003;37:735–749. doi: 10.1016/s0896-6273(03)00084-9. [DOI] [PubMed] [Google Scholar]
- 12.West AB, Dawson VL, Dawson TM. Trends Neurosci. 2005;28:348–352. doi: 10.1016/j.tins.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.El-Khodor BF, Oo TF, Kholodilov N, Burke RE. Exp Neurol. 2003;179:17–27. doi: 10.1006/exnr.2002.8047. [DOI] [PubMed] [Google Scholar]
- 14.Smith PD, Crocker SJ, Jackson-Lewis V, Jordan-Sciutto KL, Hayley S, Mount MP, O'Hare MJ, Callaghan S, Slack RS, Przedborski S, et al. Proc Natl Acad Sci USA. 2003;100:13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borta A, Höglinger GU. J Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- 16.DeGregori J. Biochim Biophys Acta. 2002;1602:131–150. doi: 10.1016/s0304-419x(02)00051-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Momma S, Delfani K, Carlén M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisén J, Janson AM. Proc Natl Acad Sci USA. 2002;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Höglinger GU, Carrard G, Michel PP, Friguet B, Hirsch EC. J Neurochem. 2003;86:1297–1307. doi: 10.1046/j.1471-4159.2003.01952.x. [DOI] [PubMed] [Google Scholar]
- 19.Field SJ, Tsai F-Y, Kuo F, Zubiaga AM, Kaelin WG, Livingston DM, Orkin SH, Greenberg ME. Cell. 1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6. [DOI] [PubMed] [Google Scholar]
- 20.Trinh E, Boutillier AL, Loeffler JP. Mol Cell Neurosci. 2001;17:342–353. doi: 10.1006/mcne.2000.0928. [DOI] [PubMed] [Google Scholar]
- 21.Rideout HJ, Wang Q, Park DS, Stefanis L. J Neurosci. 2003;23:1237–1245. doi: 10.1523/JNEUROSCI.23-04-01237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. Nature. 2002;419:367–374. doi: 10.1038/nature01034. [DOI] [PubMed] [Google Scholar]
- 23.Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frielingsdorf H, Schwarz K, Brundin P, Mohapel P. Proc Natl Acad Sci USA. 2004;101:10177–10182. doi: 10.1073/pnas.0401229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu DX, Greene LA. Neuron. 2001;32:425–438. doi: 10.1016/s0896-6273(01)00495-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.