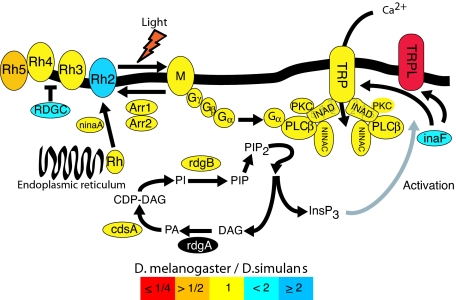
Fig. 1.
Major proteins of the phototransduction network. Colors indicate the observed relative levels of gene expression between D. melanogaster and D. simulans. Color coding indicates expression difference between species as a ratio (D. melanogaster/D. simulans). Red is ≤1/4; orange is >1/2; yellow is 1/1; light blue is <2/1; dark blue is ≥2/1; black, no data. The Drosophila phototransduction network is a paradigm for the study of signal transduction (3, 14, 23, 31, 32). The end point of the cascade is the depolarization of the photoreceptor cell, resulting from the opening of Na+- and Ca2+-permeable channels, TRP and TRPL. Light initially activates rhodopsin into active metarhodopsin, which catalyzes the activation of a heterotrimeric G protein. The Gα subunit of the activated heterotrimer then activates an effector enzyme, phosphoinositide-specific phospholipase C (PLCβ, encoded by the gene norpA), which leads to the production of two signaling molecules, DAG (diacylglycerol) and InsP3 (inositol triphosphate) from PIP2 (phosphatidylinositol-4,5-biphosphate). This ultimately leads to the activation of the light-sensitive channels TRP, TRPL, and TRPγ (the latter is not studied here). The gray arrow indicates a mechanism of action that is not totally elucidated. TRPL and TRPγ form heteromultimers with TRP and with each other. Modulation of the ratio of TRP/TRPL subunits in the rabdhomeres through the light-sensitive translocation of TRPL has been suggested as a physiological mechanism increasing sensitivity to dim background light and allowing response to a wider dynamic range of light intensities (13). A sharp response is provided by the inactivation reactions that take place soon after activation. Two important players in the inactivation are the arrestins, which bind to and inactivate rhodopsin. Activity of both the GTP-bound Gqα subunit and PLC is terminated by the GTPase activity of the G protein and other components. Light-sensitive channels are inactivated by Ca2+ through the action of calmodulin. RDGC is a rhodopsin phosphatase that is regulated by calmodulin. Its function in deactivation of the response, by means of the deactivation of rhodopsin-mediated signaling, is mostly inferred from mutant phenotypes. Many of the molecules are assembled in a signalplex whose core component is INAD, a scaffold protein with five PDZ protein-interaction modules. Other proteins interact with INAD, but their localization in the rabdhomeres does not depend on INAD. InaF serves as a regulator of the calcium-channel activity, but its mode of action is not completely elucidated. NinaA is responsible for transporting rhodopsin from the endoplasmic reticulum to the cell membrane. PKC is a protein kinase encoded by inaC, whose role is not fully understood, but mutant phenotypes suggest a role in adaptation to light and termination response.