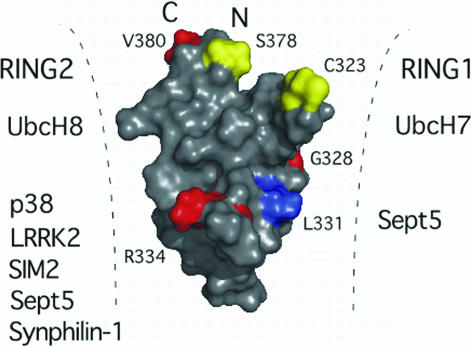
Fig. 5.
Proposed Parkin interactions and arrangement of IBR and RING domains. Surface representation of the IBR structure showing the positions of ARJP mutations (G328E, R334C, V380L) is shown in red. Two of these mutations (G328E, R334C), and the conserved residue L331 (blue) lie along the L1 loop and result in decreased binding and ubiquitination of substrates such as synphilin-1 and p38. Other substrates are listed based on biochemical experiments that indicate interaction with either the RING1–IBR or IBR–RING2 domain pairs. Residues where S-nitrosylation is proposed (C323) or phosphorylation has been identified (S378) are shown in yellow. These residues, along with the missense mutation site V380 lie at the crown of the IBR domain where the N and C termini are close in space bringing the RING1 and RING2 domains within close proximity to each other and the IBR domain.