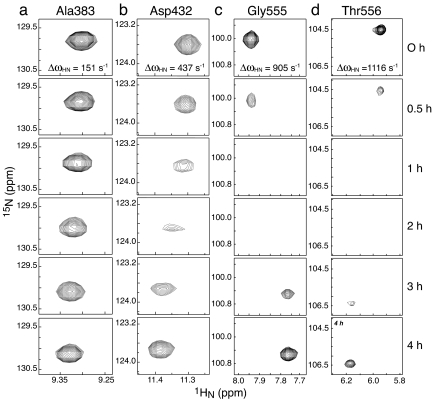
Fig. 5.
Time course of selected 1HN/15N cross-peaks in 1H-15N HSQC spectra of wild-type IIBAMtl during active intramolecular phosphoryl transfer subsequent to the depletion of PEP. (a) Ala-383; (b) Asp-432; (c) Gly-555; (d) Thr-556. The spectrum at 0 h, taken at the point of complete depletion of PEP, reflects the biphosphorylated state in which both the A and B domains are fully phosphorylated; the spectrum at 4 h represents fully dephosphorylated IIBAMtl. The effects of chemical exchange arising from reversible phosphoryl transfer between the A and B domains in species that are monophosphorylated are apparent in the spectra taken at intermediate times. The ΔωHN values were obtained from the chemical-shift differences between the spectra taken at 0 and 4 h (1H frequency of 800 MHz).