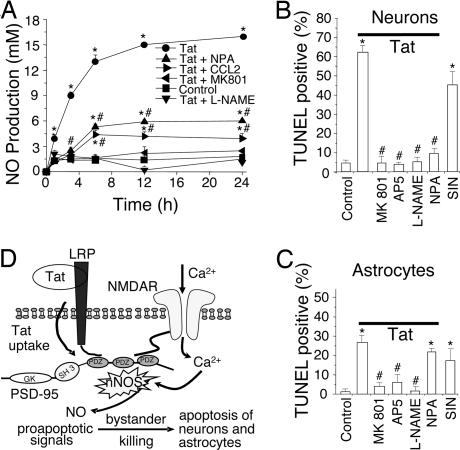
Fig. 4.
Tat induces NO production, primarily through nNOS activation, resulting in neuron and astrocyte apoptosis. (A) Cultures were treated with tat (●) or tat plus NPA (▴), CCL2 (▶), MK801 (◀), or L-NAME (▾). At different time points, medium was collected and NO production was measured by the Griess reaction. Tat treatment induced significant NO production over the untreated condition (■; P < 0.001). MK801 or L-NAME abolished tat-induced production of NO (P < 0.001 vs. tat alone). NPA or CCL2 reduced tat-induced production of NO (P < 0.001) but not to basal levels, suggesting a source of NO in addition to nNOS. The addition of MK801, L-NAME, NPA, or CCL2 alone did not change basal NO production (data not shown). ∗, P < 0.001 vs. control; #, P < 0.001 for a treatment compared with tat alone (n = 4). (B and C) Summary data of apoptosis in neurons and astrocytes after 24-h treatment. Tat induced a high percentage of apoptosis compared with control (first two bars on the left). The NMDA blockers, MK801 and AP5, provided substantial protection (third and fourth bars). The general NOS antagonist, L-NAME, blocked apoptosis in both cell types. The nNOS-specific blocker, NPA, greatly inhibited neuronal apoptosis with little effect on astrocyte apoptosis (sixth bar). The NO donor, SIN, alone (seventh bar) induced almost as much apoptosis as tat [∗, P < 0.001 vs. control conditions and #, P < 0.001 compared with tat treatment (n ≥ 6)]. (D) Proposed mechanism of tat-induced apoptosis. Tat induces formation of a macromolecular complex of LRP, PSD-95 [protein with three PDZ domains, an SH3 and guanylyl kinase (GK)-like domain], NMDAR, and nNOS. The formation of this complex generates proapoptotic signals, such as NO, that can be transmitted to cells lacking NMDARs, resulting in extensive apoptosis.