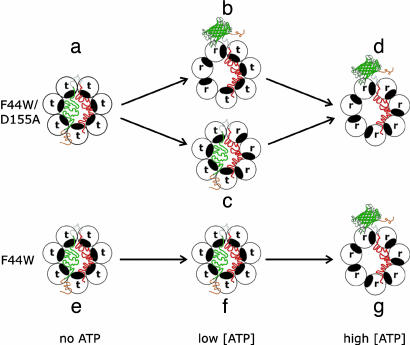
Fig. 4.
Scheme showing coupling between ATP-induced conformational changes in GroEL and release and folding of EGFP in the EGFP–rhodanese chimera. The ATP-induced conformational change of a subunit from the t state (with low affinity for ATP and high affinity for nonfolded substrates) to the r state (with high affinity for ATP and low affinity for nonfolded substrates) is represented by a counterclockwise rotation that causes the protein substrate-binding site (black) to face away from the cavity. The wild-type variant (F44W) of GroEL and the F44W/D155A mutant undergo ATP-induced concerted (t7→r7) and sequential (e.g., t7→t4r3→r7) allosteric transitions, respectively. The color-coding of the unfolded and folded (ribbon diagram of EGFP) parts of the chimera are according to Fig. 1A. For simplicity, only the substrate-bound ring of GroEL is shown in this scheme.