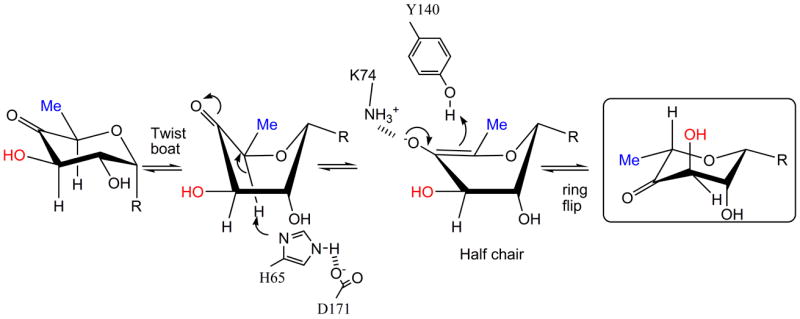
Figure 6.
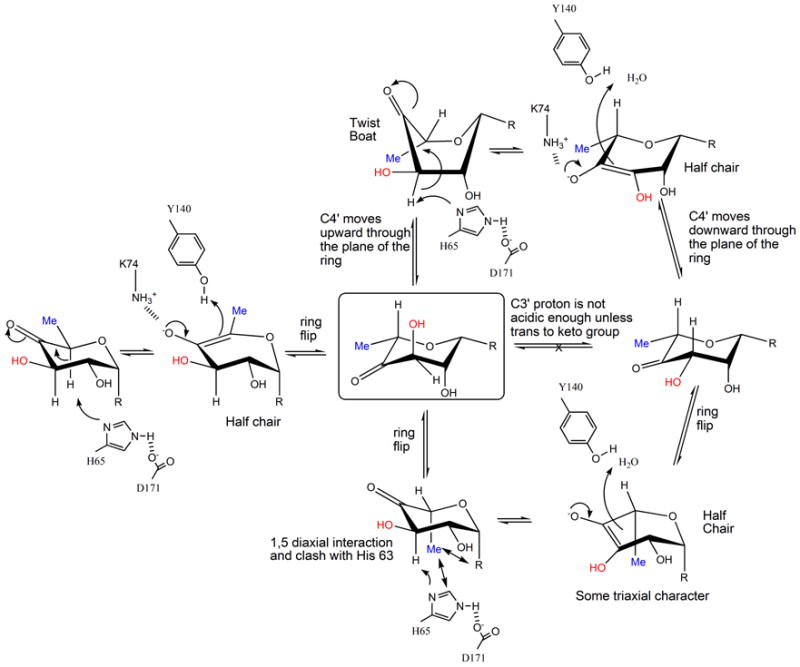
(a) R=OdTDP. A possible mechanism for RmlC based on structural and biochemical data. The key active site residues are shown, the H65 is the catalytic base for both epimerizations, K73 stabilizes the enolate and Y134 acts as the acid for the first epimerization. The mono-epimerized intermediate is shown boxed and has the equatorial linkage between carbohydrate ring and nucleotide. It cannot proceed directly to product because the C3′ proton is only sufficiently acidic when it is orthogonal to the plane of the carbonyl function.
(b) R=OdTDP. An alternative route for the first epimerization using a twist boat form of substrate, the mono epimerized intermediate is shown boxed. The apparent preference of RmlC for the equatorial linked sugar nucleotide suggests this is a possibility.
