Abstract
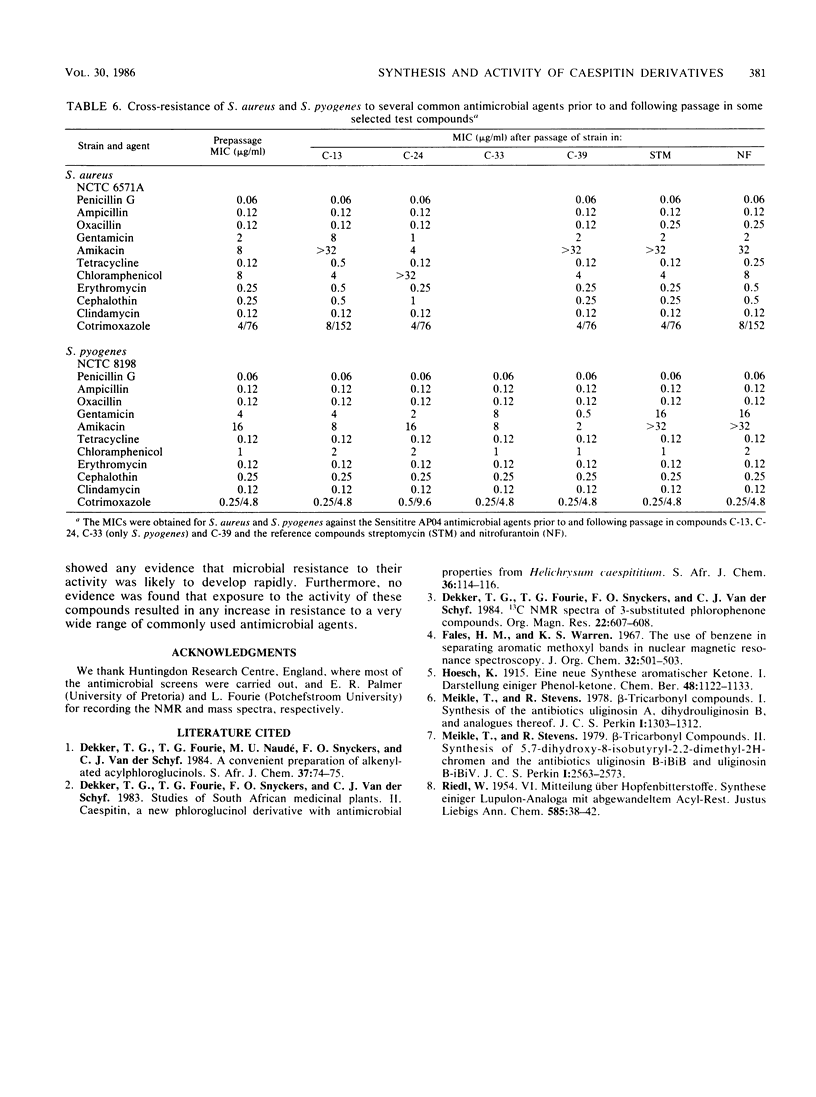
Chemical modification of the naturally occurring phlorophenone antimicrobial agent caespitin is described. These modifications include variations in the phenone side chain, substitution with prenyl, allyl, and benzyl in the 4-position of the phlorophenone nucleus, and ring cyclizations via etherification to give furan and chroman compounds. Several of these derivatives show enhanced in vitro potency over caespitin. Studies on the development of microbial resistance against these compounds show that no or very little resistance developed after several passes of these compounds in representative microbial strains.
Full text
PDF