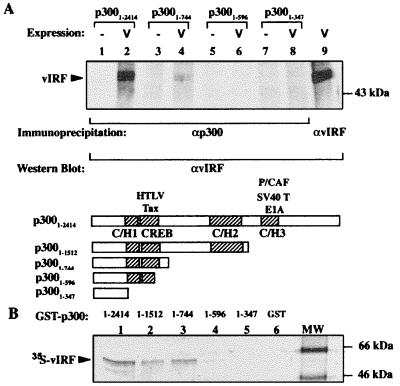
Figure 2.
Protein–protein interactions between p300 and vIRF. (A) Western blot for vIRF coimmunoprecipitated with N-15 anti-p300 (αp300) antibody from IRF1/2−/− MEF cells transiently overexpressing vIRF and p300. vIRF is detected by rabbit polyclonal antibodies as a ≈50-kDa band in lane 2 (full-length p3001–2414) and in lane 4 containing a truncated p300 fragment missing C/H2 and C/H3 domains (p3001–744). Shorter constructs (lane 6, p3001–596, and lane 8, p3001–347) failed to coimmunoprecipitate vIRF. Lanes 1, 3, 5, and 7 are immunoprecipitations without p300 overexpression, and lane 9 shows vIRF protein for comparison. (B) Autoradiograph of GST-p300 pull-down assay for interaction with vIRF. The GST-p300 fusion proteins were precipitated on glutathione beads in the presence of in vitro translated [35S]methionine-labeled vIRF. vIRF binds to GST-p3001–2616, GST-p3001–1512, and GST-p3001–744 (lanes 1–3) but not to shorter p300 fragments (GST-p3001–596, lane 4, or GST-p3001–347, lane 5). Lane 6 contains the GST control protein alone.