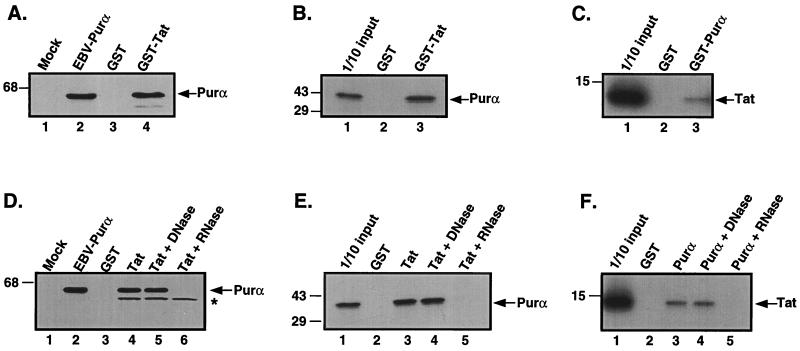
Figure 1.
HIV Tat and Purα associate with each other via RNA. (A) Extract containing a histidine-tagged Purα was incubated with either GST (lane 3) or GST-Tat fusion proteins (lane 4) attached to glutathione Sepharose resin. (B) In vitro translated [35S]labeled Purα was incubated with either GST (lane 2) or GST-Tat fusion proteins (lane 3). (C) In vitro translated [35S]labeled Tat protein was incubated with either GST (lane 2) or GST-Purα fusion proteins (lane 3). For A, B, and C, incubation was performed for 1 h at 4°C, and the resin was subsequently pelleted and washed with binding buffer. Bound proteins were eluted by boiling in Laemmli sample buffer and, after separation by SDS/PAGE, were detected by either immunoblot analysis with T7 antibody (A) or autoradiography (B and C). An amount equivalent to 10% of the material used for the assays was applied to the input lane in B and C. The position of the molecular mass markers are indicated on the left of the gels. The arrow indicates the position of histidine tagged Purα (A), [35S]labeled in vitro translated Purα (B), and [35S]labeled in vitro translated Tat (C). (D) Extract containing a histidine-tagged Purα was incubated with GST (lane 3), GST-Tat (lane 4), or GST-Tat in the presence of DNase (lane 5) and RNase (lane 6). (E) In vitro translated [35S]labeled Purα was incubated with GST (lane 2), GST-Tat (lane 3), or GST-Tat in the presence of DNase (lane 4) and RNase (lane 5). (F) In vitro translated [35S]labeled Tat was incubated with GST (lane 2), GST-Purα (lane 3), or GST-Purα in the presence of DNase (lane 4) and RNase (lane 5). The position of molecular mass markers are indicated on the left of each gel. Arrows indicate the position of histidine-tagged Purα (D), in vitro translated Purα (E), and in vitro translated Tat (F). The asterisk indicates a nonspecific cross reacting band.