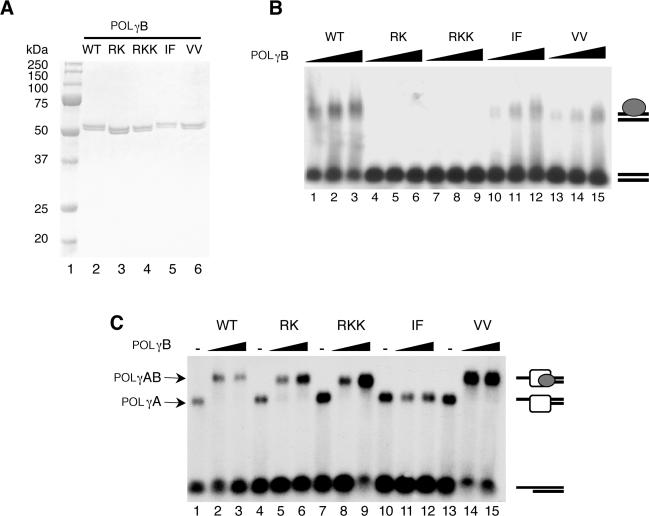
Figure 1.
Purification and characterization of the recombinant POLγB proteins (A), Purified recombinant wt and mutant versions of POLγB (0.5 µg) were separated by SDS–PAGE (12.5%) and revealed with Coomassie brilliant blue staining. lane 1, size marker; lane 2, wt POLγB; lane 3, RK mutant; lane 4, RKK mutant; lane 5, IF mutant; lane 6, VV mutant. (B) DNA binding affinity of POLγB mutants was determined by EMSA using a P32-labeled 50-bp dsDNA probe (10 fmol/reaction). The reactions were performed as described in experimental procedures in the presence of increasing amount of the indicated POLγB versions (0.2, 0.4 and 0.8 pmol). After incubation for 10 min at RT the protein-DNA complexes were analyzed on a 0.8% native agarose gel. (C) Interactions between the POLγB mutants and the catalytic POLγA subunit were determined by EMSA using a P32-labeled primed DNA template (10 fmol). The template was incubated with POLγA (0.5 pmol) and increasing amounts of POLγB (0, 0.5 and 1 pmol) for 10 min at RT. The protein–DNA complexes were immediately analyzed on a native 0.8% agarose gel.