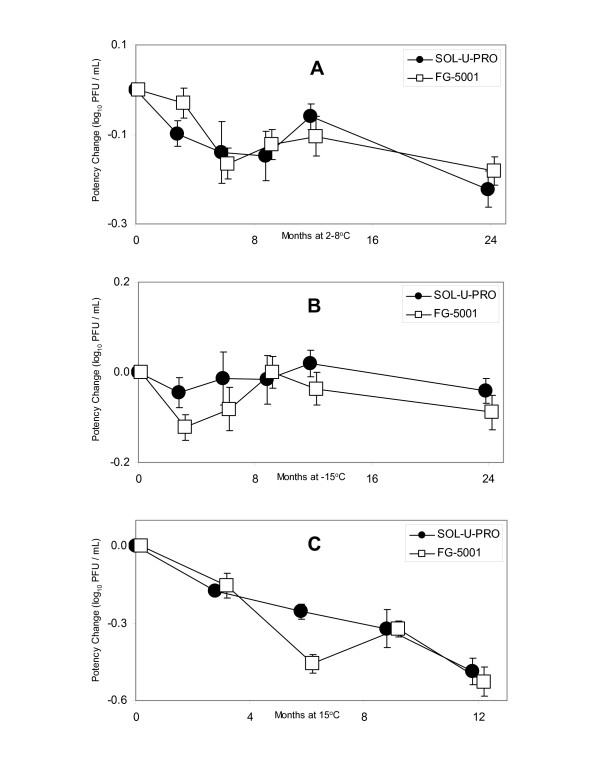
Figure 1.
The long-term stability of VZV (Oka/Merck) formulated in hydrolyzed porcine gelatin (SOL-U-PRO), or recombinant human gelatin (FG-5001) stabilized varicella vaccine matrices. The VZV (Oka/Merck) potency change was determined as a difference between the potency of control samples stored at -70°C, and samples stored at 2–8°C (Figure 1A), and -15°C (Figure 1B) for 24 months, and 15°C (Figure 1C) for 12 months, respectively. The value of 0 for the potency loss (change) at time interval 0 months represents the stability model starting at that point. Vaccine samples were analyzed by the VZV plaque assay in format 1 × 12. The VZV potency change is in log10 PFU/mL.