FIG. 7.
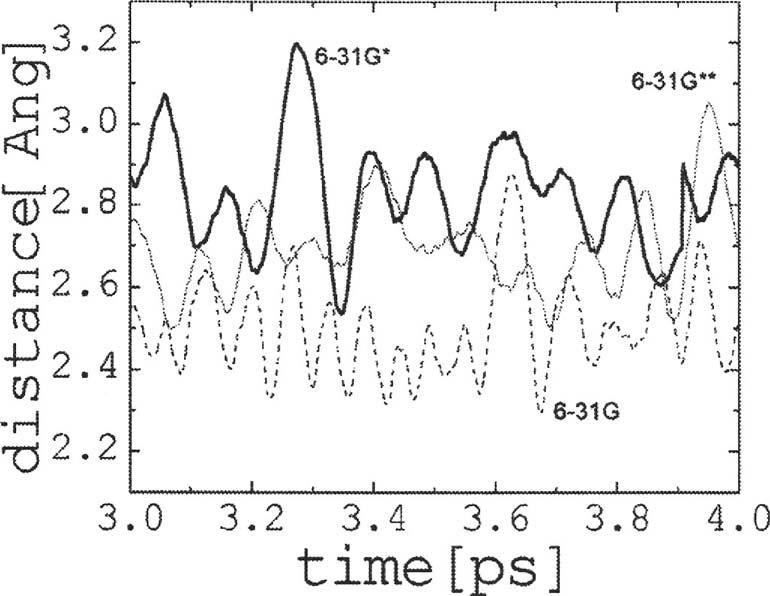
Oxygen-oxygen distance between water molecules W4 and W5 for the last 1 ps of simulation in the nonperturbed system calculated with the 6-31G, 6-31G*, and 6-31G** basis sets. Lack of polarization shortens the LO distances between contiguous water molecules, reflecting stronger water-water interactions along the chain. Introducing polarization on the hydrogen atoms and/or heavy atoms leads generally to largerLO distances.
