FIGURE 6. High affinity binding of CLIC-5b by immobilized Src SH2 or SH3 domains and co-immunoprecipitation of kinase activity.
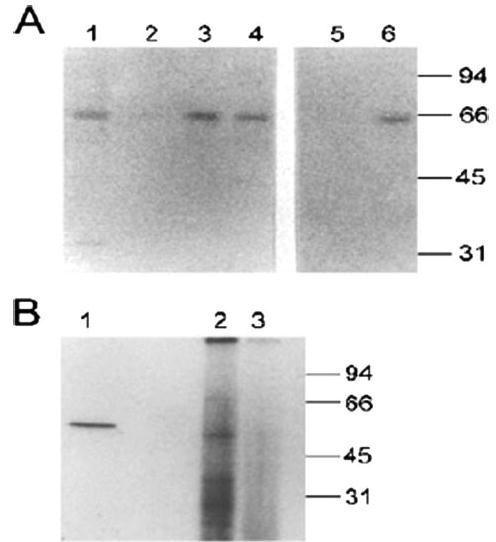
A, retention of CLIC-5b by immobilized SH2 and SH3 domains of Src. Twenty micrograms of solubilized osteoclast membranes (lane 1) or those proteins which bound to immobilized GST (control; lane 2), GST-srcSH2 fusion protein (lanes 3 and 5) or GST-srcSH3 fusion protein (lanes 4 and 6) were separated and probed with affinity-purified antibody AP656. Samples shown in lanes 5 and 6 were treated with alkaline phosphatase prior to binding to immobilized fusion protein. B, 300 μg of solubilized membrane protein from cultured in vitro differentiated osteoclasts were immunoprecipitated by preimmune sera (lane 3) or antisera 656 (lane 2) as described under “Materials and Methods.” The washed immunoprecipitates were incubated in kinase reaction media with [γ-32P]ATP and then separated by SDS-PAGE and labeled bands detected by autoradiography. In lane 1, 1 μg of purified human Src was incubated in the kinase reaction mixture before separation and autoradiography. Migration positions of molecular mass standards are shown in kilodaltons.
