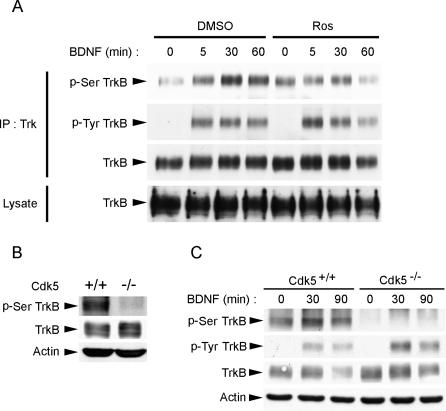
Figure 3. Ser478 Phosphorylation of TrkB Required Cdk5 Activity In Vivo.
(A) BDNF stimulation resulted in an increase in p-Ser478 TrkB (p-Ser TrkB) levels in cortical neurons. Treatment with Cdk5 selective inhibitor Ros (25 μM) inhibited the BDNF-induced increase in p-Ser478 TrkB, although Ros treatment also resulted in a slight increase in basal p-Ser478 TrkB.
(B) cdk5 +/+ and cdk5 −/− brain lysates were immunoblotted against TrkB, phospho-TrkB at Ser478, and β-actin as loading control. p-Ser478 TrkB was almost completely absent in cdk5 −/− brain, indicating the importance of Cdk5 in the phosphorylation of TrkB at Ser478 in vivo.
(C) Cortical neurons isolated from cdk5 +/+ and cdk5 −/− brain were treated with BDNF for different periods. Interestingly, while BDNF enhanced TrkB Ser478 phosphorylation in cdk5 +/+ cortical neurons, TrkB Ser478 phosphorylation was not detected in cdk5 −/− neurons, nor did BDNF stimulation enhance Ser478 phosphorylation, indicating that BDNF-stimulated increase in TrkB Ser478 phosphorylation requires Cdk5 activity.