Fig. 1. Trypsinized AAV2 capsids generate novel fragments. A. Trypsin used during purification.
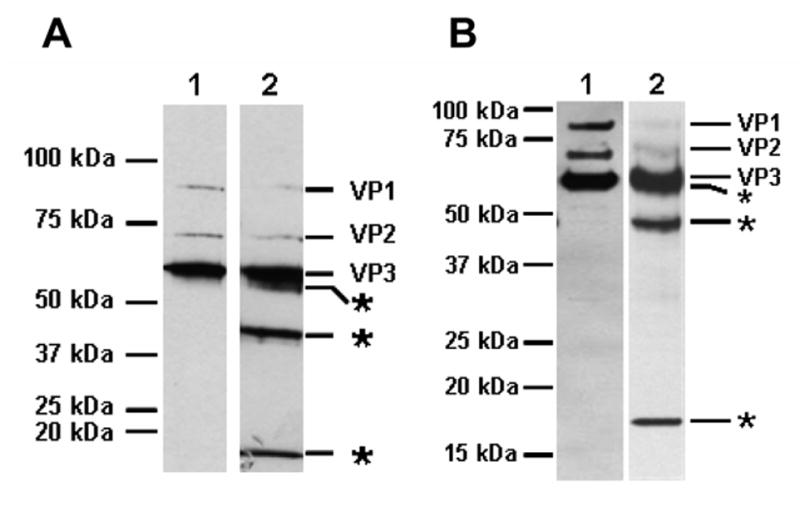
Lane 1, AAV2-GFP cell lysates made in the presence of deoxycholate (DOC) and centrifuged on CsCl density gradients (DOC/CsCl). Lane 2, AAV2-GFP cell lysates made in the presence of 0.02% trypsin and DOC (30 minutes at 37°C), and purified on CsCl density gradients (Trypsin/DOC/CsCl). The peak fraction of full capsids was denatured and separated on a 10% SDS-PAGE gel, blotted, and probed with anti-AAV polyclonal antisera. B. Trypsinization of purified AAV2. Purified rAAV2-GFP virions (F-T/iodixanol/Heparin, Lane 1) were treated with 0.02% trypsin for 24 hours (Lane 2), and the capsid proteins were separated on a 12.5% SDS-PAGE gel, blotted and probed with anti-AAV2 polyclonal antisera. The positions of molecular weight standards are indicated on the left side and the tryptic fragments are indicated on the right side with asterisks.
