Abstract
Xenobiotic-metals such as mercury (Hg) and silver (Ag) induce an H-2 linked antinucleolar autoantibody (ANolA) production in susceptible mice. The mechanism for induction of ANolA synthesis is not well understood. However, it has been suggested that both metals interact with nucleolar proteins and reveal cryptic self-peptides to nontolerant autoreactive T cells, which in turn stimulate specific autoreactive B cells. In this study, we considered this suggestion and asked if mercury and silver display, if not identical, similar cryptic self-peptides, they would induce comparable ANolA responses in H-2 susceptible mice. We analysed the development of ANolA production in mercury- and/or silver-treated mice of H-2s, H-2q and H-2f genotypes. We found that while mercury stimulated ANolA synthesis in all strains tested, silver induced ANolA responses of lower magnitudes in only H-2s and H-2q mice, but not in H-2f mice. Resistance to silver in H-2f mice was independent of the dosage/time-period of silver-treatment and non-H-2 genes. Further studies showed that F1 hybrid crosses between silver-susceptible A.SW (H-2s) and -resistant A.CA (H-2f) mice were resistant to silver, but not mercury with regard to ANolA production. Additionally, the magnitudes of mercury-induced ANolA responses in the F1 hybrids were lower than those of their parental strains. The above differential ANolA responses to mercury and silver can be explained by various factors, including the different display of nucleolar cryptic peptides by these xenobiotics, determinant capture and coexistence of different MHC molecules. Our findings also suggest that the ability of a xenobiotic metal merely to create cryptic self-peptides may not be sufficient for the induction of an ANolA response.
Keywords: anti-nucleolar antibodies, fibrillarin, H-2 genotype, mercury, silver
Introduction
Chemically induced autoimmune manifestations in laboratory animals have been used as experimental models to study systemic autoimmune diseases (reviewed in [1–3]). Among these models, murine mercury-induced autoimmunity has gained special interest. This is chiefly because autoantibodies with certain specificities can be induced in this model easily and with high reproducibility. The most specific autoantibodies, which are produced after chronic exposure to subtoxic doses of mercury in susceptible mice, are antinucleolar autoantibodies (ANolA) [4,5]. These autoantibodies react with fibrillarin [6–8], a 34–36 kDa protein which is associated with U3, U8, U13, U14, X and Y small nucleolar RNAs in vertebrates [9]. Synthesis of ANolA is probably the most striking feature of murine mercury-induced autoimmunity and possesses three peculiarities. First, its induction requires T cells, particularly T-helper (CD4+) cells [10]. Secondly, mercury-induced ANolA share several similarities in binding to fibrillarin with that of ANolA found with high titres in sera of some patients with scleroderma [8, 11, 12]. Thirdly, its development is under strict control of H-2 genotype, i.e. only mouse strains of H-2s, H-2q and H-2f genotypes irrespective of their non-H-2 genes produce ANolA in response to mercury [13, 14, 15, 16, 17, 18, 19]. By using intra-H-2 recombinant mouse strains, susceptibility to mercury-induced ANolA production could be mapped to the I-A loci of H-2 class II genes [15]. It has been shown that the other H-2 class II locus (I-E) either suppressed [14] or did not influence the mercury-induced ANolA response [15]. These features make the murine model of mercury-induced ANolA production suitable for studying the mechanism by which environmental factors can trigger MHC associated specific autoimmune responses.
In the past few years, studies have shown that another metal, silver (Ag), could also induce ANolA/antifibrillarin autoantibodies (AFA) in mercury-susceptible mouse strains which carried I-As genotype, e.g. SJL, A.SW, B10.S and A.TH [20–22]. Interestingly, in most cases, silver-induced ANolA production resembled that of mercury-induced ANolA [20–22]. This, and the fact that mercury and silver as xenobiotic metals are able to form strong chemical bonds with organic donors [23], led the investigators to propose a general mechanism for induction of ANolA production by mercury [2] and silver [22] in genetically susceptible mice. According to this proposition, mercury, silver and possibly other heavy metals by having high affinity for different organic donors bind tightly to several protein side-chains creating stable metal-protein complexes. Formation of metal–protein (here mercury–, silver–fibrillarin) complexes results in an incomplete protein unfolding. Further enzymatic cleavage of this incomplete unfolded protein would lead to the creation of cryptic self-peptides capable of binding to the MHC (H-2 in the mouse) class II molecules in susceptible mice [2,22]. Metal-induced presentation of cryptic self-peptides induces an activation in nontolerant nucleolar specific T cells, which in turn elicit an ANolA response [2,22]. Based on this suggestion, we deduced that if exposure to mercury and/or silver would result in displaying of similar or identical cryptic nucleolar peptides, mouse strains with susceptible H-2 genotypes (H-2s, H-2q and H-2f) would respond to these xenobiotics correspondingly (if not equally). In the present study, we performed experiments to verify our deduction.
Materials and methods
Mice
Female B10.S (H-2s), B10.G (H-2q) and B10.M (H-2f) mice were purchased from Harlan (Harlan Olac Ltd, Bicester, UK). Female FVB/N (H-2q) mice were obtained from M & B A/S (M & B A/S, Ry, Denmark). Breeding couples of A.CA (H-2f) and A.SW (H-2s) were obtained originally from the animal facilities at the Microbiology and Tumor Biology Center (MTC), the Karolinska Institute, Stockholm, Sweden. These strains, as well as (female A.SW × male A.CA) F1 and (female A.CA × male A.SW) F1 hybrids, were then produced further and kept in the animal facilities of the Department of Immunology, Stockholm University. The mice were kept under 12-h dark and 12-h light cycles and were given food pellets and tap water ad libitum. All mice were 4–8 weeks old at the beginning of each experiment.
HgCl2 and AgNO3 treatment
A solution of 0·4 mg/ml HgCl2 (analytical grade, Merck, Darmstadt, Germany) was prepared in sterile 0·9% NaCl solution. A solution of 0·573 mg/ml AgNO3 (analytical grade, Merck, Darmstadt, Germany) was prepared in sterile water. Groups of the different mouse strains as well as F1 hybrids (4–13 mice/group as indicated in the figures or figure legends) were injected subcutaneously (s.c.) with either 0·1 ml of HgCl2 (1·6 mg/kg body weight) or AgNO3 (2·5 mg/kg body weight) solutions every third day for 4 weeks. Control groups received 0·1 ml of sterile 0·9% NaCl by s.c. route.
In a dose–response experiment, different groups (four mice per group) of B10.M (H-2f) and FVB/N (H-2q) mice were injected s.c. with 0·1 ml AgNO3 solutions of different doses (2·5, 5·0 and 7·5 mg/kg body weight) every third day for 4 weeks (FVB/N mice) or 8 weeks (B10.M mice). Control groups received 0·1 ml of sterile 0·9% NaCl solution by the s.c. route.
Blood collection and serum preparation
At the end of each treatment period, the mice were bled by retroorbital puncture under light methophane anaesthesia. Thereafter the mice were killed by cervical dislocation. The blood from each mouse was allowed to clot at 4°C and serum was separated after centrifugation. The sera were kept at − 20°C until tested for antibody/autoantibody content.
Detection of ANolA by indirect immunofluorescence (IIF)
The presence of IgG1, IgG2a, IgG2b and IgG3 ANolAs in sera was determined by an indirect immunofluorescence method as described previously [18,19]. Briefly, rat-liver sections or HEp-2 cells grown as monolayers on slides were used as substrates and FITC-conjugated goat antimouse IgG (Cedarlane Laboratories Limited, Ontario, Canada), IgG1, IgG2a, IgG2b and IgG3 as detection antibodies (Southern Biotechnology, Birmingham, AL, USA and/or Caltag Laboratories CL, USA) (the sensitivity of FITC-conjugated antibodies from these two different sources were compared and found to be comparable). The pattern and titres of ANolA were then assessed under a Reichard-Jung Polyvar microscope (Vienna, Austria). The initial dilution for the sera was 1 : 50. When at this dilution no specific green fluorescence was detected, the result was recorded as ‘0’ (zero). The highest dilution of mouse sera at which nucleolar fluorescence could be detected was defined as the titre of IgG, IgG1, IgG2a, IgG2b and/or IgG3 ANolA.
Detection of ANolA by immunoblotting
For immunoblotting, nuclear extract was prepared from HEp-2 cells as described by Behrens et al. [24]. Briefly, Hep-2 cells (ATCC, Rockville, MD, USA) grown in MEMEagle with l-glutamine and Earle's BSS medium (Gibco/BRL, Life Technologies AB, Täby, Sweden), containing 1·0 mm sodium pyruvate, 0·1 mm non-essential amino acids, penicillin (50 units/ml)/streptomycin (50 µg/ml) and 10% fetal calf serum (FCS) were treated with trypsin and harvested by centrifugation and re-suspended in PBS-Earle and centrifuged again to obtain a cell pellet. The cell pellet was resuspended in buffer A (10 mm Hepes-KOH, pH 8, 10 mm KCl, 1·5 mm MgCl2, 0·5 mm dithioerythrithol) and incubated on ice for 10 min. The cells were then centrifuged again and the cell pellet was resuspended in buffer A and thereafter the cells were broken by 10 strokes in a 40-ml douncer. The cytoplasmic membrane was removed by two successive centrifugations in a JA-20 rotor for 10 min at 1000 g and then 20 min at 25 000 g. The obtained pellet (cell nuclei) was resuspended in buffer C (20 mm Hepes-KOH, pH 8, 420 mm NaCl, 1·5 mm MgCl2, 0·5 mm dithioerythrithol, 0·5 mm PMSF, 0·2 mm EDTA, pH 8, 25% v/v glycerol). The nuclei were broken by 10 strokes in a 40-ml douncer. The resulting suspension was stirred on ice for 30 min. The nuclear membrane was removed by centrifugation in a JA-20 rotor at 25 000 g for 30 min. The nuclear extract was dialysed with buffer G (20 mm Hepes-KOH, pH 8, 150 mm KCl, 1·5 mm MgCl2, 0·5 mm dithioerythrithol, 0·5 mm PMSF, 0·2 mm EDTA, pH 8, 5% v/v glycerol).
Seventy-five µl of the nuclear extract was mixed with 250 µl of sample buffer containing 2ME and boiled for 5 min [25] and then subjected to electrophoresis on 12% SDS polyacrylamide gel and transferred to a nitrocellulose membrane at 250 mA for 1 h in 25 mm Tris (pH 8·3), 192 mm glycine and 20% methanol [26]. The nitrocellulose membrane was saturated in TBS (Tris-buffered saline) containing 5% non-fat dry milk at room temperature (RT) for 14 h. The nitrocellulose membrane was then washed with TTBS (TBS + 0·05% Tween) and cut into strips and incubated with the sera of mercury-, silver-, and saline-injected mice diluted 1 : 100 in TTBS containing 1% non-fat dry milk (TTBS−1% milk) at RT for 14 h. After two washes with TTBS, the bound antibodies on the strips were detected with alkaline phosphatase-conjugated goat antimouse IgG1 (Southern Biotechnology, Birmingham, AL, USA) diluted 1 : 2500 in TTBS−1% milk and incubated at RT for 2 h. After three successive washes with TTBS (twice) and TBS (once), the strips were exposed to the colour developer, AP-Purple (Intergen, MA, USA).
Statistical analysis
Serum titres of IgG1, IgG2a, IgG2b and IgG3 ANolA were shown as the mean + 1 s.e. (standard error). We estimated s.e. because it represents the expected standard deviation of the statistic in the case where a large number of samples (here animals) had been used. The difference between mercury-, silver- and saline-injected animals for these parameters was subjected to statistical analysis using the Wilcoxon–Mann–Whitney test. The differences between the mice, which were injected with different doses of silver for the above-mentioned parameters, were subjected to statistical analysis using the multiple comparisons test. All analyses were performed using WinSTAT software (R. Fitch Software, Medma AB, Vänerborg, Sweden).
Results
Differential mercury- and silver-induced ANolA production in susceptible mice
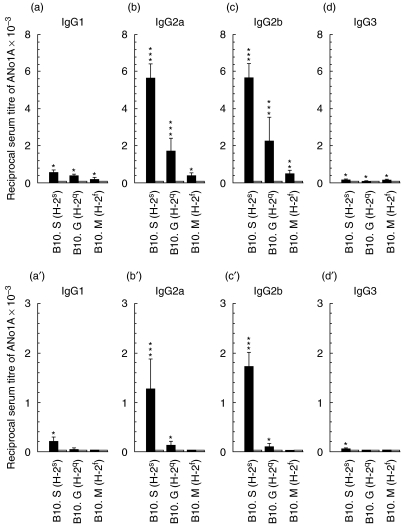
In order to test whether mercury and silver use similar mechanism(s) to induce ANolA production, groups of H-2-congenic mouse strains on a B10 genetic background (B10.S, B10.G and B10.M), which carried the permissive H-2 genotypes for ANolA production (H-2s, H-2q and H-2f, respectively) were injected continuously with mercury, silver and/or as controls, with saline for 4 weeks. At the end of the experiment, the sera were tested for the presence of ANolA of different IgG isotypes by using an IIF technique. As shown in Fig. 1a, and Fig. 2b,d,f, mercury was able to induce ANolA production of different IgG isotypes (mainly IgG2a and IgG2b and slightly IgG1) in all H-2-congenic strains tested as compared to the saline-injected controls. However, the magnitude of mercury-induced ANolA synthesis varied among the H-2-congenic strains, i.e. the highest and lowest serum titres of IgG (different isotypes) ANolA were found in B10.S (H-2s) and B10.M (H-2f) mice, respectively (Fig. 1a). Mercury-treated B10.G (H-2q) mice exhibited an intermediate increase in the serum levels of IgG1, IgG2a and IgG2b ANolA (Fig. 1a).
Fig 1.
Induction of antinucleolar autoantibodies (ANolAs) of various IgG isotypes in H-2 congenic B10 mice injected with mercuric chloride (HgCl2) and silver nitrate (AgNO3). Groups of female B10.S (H-2s), B10.G (H-2q) and B10.M (H-2f) mice were injected repeatedly subcutaneously (s.c.) with HgCl2 (solid bars, upper panel) or AgNO3 (solid bars, lower panel) and/or NaCl (open bars) for 4 weeks. At the end of each experiment the mice were bled and killed. The sera were tested for the presence of ANolA by using an indirect immunofluorescence (IIF) method. Rat liver sections were used as substrates. Data are shown as mean + 1 se. Significant differences between the parameters in mercury- or silver- and saline-injected mice were calculated by Wilcoxon–Mann–Whitney test. *P < 0·05; **P < 0·01; ***P < 0·001. N.T.: not tested.
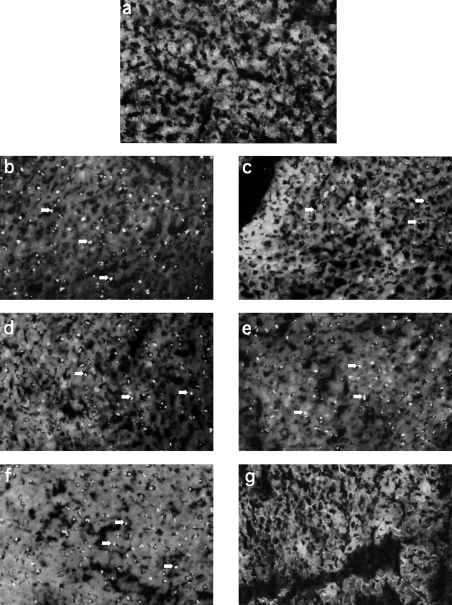
Fig 2.
Anti nucleolar autoantibody (ANolA) pattern in mercury- and silver-injected B10.S (H-2s), B10.G (H-2q) and B10.M (H-2f) mice. Sera collected from mercury- (left panel, b, d and f) and silver-injected (right panel, c, e, g) B10.S (H-2s)(b-c), B10.G (H-2q) (d,e) and B10.M (H-2f) (f,g) mice, as described for Fig. 1 were tested for IgG2a ANolA using an indirect immunofluorescence (IIF) method. A serum collected from a saline-injected mouse (a) was used as the negative control. Rat liver sections were used as substrates. Arrows indicate the clumpy nucleolar staining in the responder mice. Magnification × 400. Serum dilution 1 : 200.
Compared to mercury, silver was only able to induce ANolA production in B10.S (H-2s) and B10.G (H-2q), but not in B10.M (H-2f) mice (Fig. 1a′) and (Fig. 2c,e,g). In addition, treatment with silver resulted in the development of lower serum titres of IgG2a and IgG2b ANolA in B10.S (H-2s) and B10.G (H-2q) mice (Fig. 1b′) as compared with mercury-treatment in identical mouse strains (Fig. 1b). Because, in response to silver, B10.S and B10.G produced only low levels of ANolA (compared to mercury), it was possible that the ANolA levels in mercury- and silver-treated B10.M (H-2f) mice were below detection. To rule out this possibility, we measured the serum levels of IgG ANolA in these mice by a panreactive anti-IgG (H + L) reagent. While mercury-injected B10.M (H-2f) mice exhibited high titres of IgG ANolA in their sera, none of the silver-injected B10.M (H-2f) mice showed any detectable IgG ANolA in their sera (not shown). These results show that mercury and silver stimulate ANolA production differentially in H-2 susceptible mice and that H-2f genotype is a resistant genotype for silver-induced ANolA production.
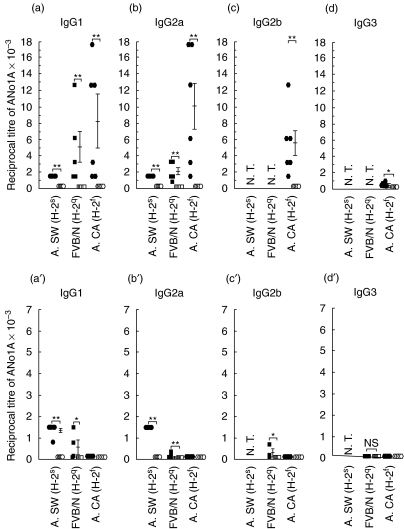
Studies on mercury- and silver-induced ANolA production have shown that B10 genes (non-H-2 genes) negatively influenced the susceptibility to ANolA synthesis [13, 16, 18]. Therefore, it was possible that B10 non-H-2 genes rather than H-2f genes conferred the non-responsiveness to silver-induced ANolA synthesis in B10.M mice. To rule out this possibility, mercury- and silver-induced ANolA production were tested in mice carrying susceptible H-2 genotypes for ANolA synthesis on other background (non-H-2) genes such as FVB/N and A. Groups of A.SW (H-2s), FVB/N (H-2q) and A.CA (H-2f) mice were treated with mercury, silver and/or saline for 4 weeks. At the end of the experiment, the sera were tested for the presence of ANolA of different IgG isotypes by using an IIF technique. Again, as shown in Fig. 3a, mercury induced high serum titres of both IgG1 and IgG2a ANolA, in all tested mouse strains. The serum titres of mercury-induced IgG1 and IgG2a ANolA in A.CA (H-2f) mice were higher than those in A.SW (H-2s) (which are H-2-congenic with A.CA) and FVB/N (H-2q) mice (Fig. 3a). High titres of IgG2b ANolA and low levels of IgG3 ANolA were also found in the sera of mercury-injected A.CA mice (H-2f) (Fig. 3c). These findings demonstrate that in addition to H-2s and H-2q genotypes, H-2f genotype is also a highly susceptible genotype for mercury-induced ANolA production.
Fig 3.
Induction of antinucleolar autoantibodies (ANolAs) of various IgG isotypes in mercury- and silver-treated A.SW (H-2s), FVB/N (H-2q) and A.CA (H-2f) mice. Groups of female A.SW (H-2s) (solid and open circles), FVB/N (H-2q) (solid and open squares) and A.CA (H-2f) (solid and open polygons) mice were injected repeatedly subcutaneously (s.c.) with HgCl2 (solid symbols, upper panel) or AgNO3 (solid symbols, lower panel) and/or NaCl (open symbols upper and lower panels) for 4 weeks. At the end of each experiment the mice were bled and killed. The sera were tested for the presence of ANolA using an indirect immunofluorescence (IIF) method. Hep-2 cell line was used as the substrate. Data are shown as mean + 1 s.e. Significant differences between the parameters in mercury- or silver- and saline-injected mice were calculated by Wilcoxon–Mann–Whitney test. *P < 0·05; **P < 0·01; ***P < 0·001.N.T.: not tested.
The development of silver-induced ANolA production in A.SW (H-2s) and FVB/N (H-2q) and A.CA (H-2f) mice was very similar to that observed in B10 H-2-congenic mice. Again, silver-injected mice with H-2s (A.SW) and H-2q (FVB/N) genotypes exhibited high serum titres of IgG ANolA (here IgG1 and IgG2a isotypes), whereas none of the mice with H-2f (A.CA) genotype showed any detectable IgG ANolA (IgG1, IgG2a, IgG2b and IgG3 isotypes) in their sera (Fig. 3a′). This was despite the finding that A.CA (H-2f) mice were highly susceptible to mercury-induced ANolA production (Fig. 3a). As in H-2 congenic B10 mice, silver-treated A.SW (H-2s) and FVB/N (H-2q) mice exhibited lower serum titres of IgG ANolA (Fig. 3a′) compared with those treated with mercury (Fig. 3a). These results confirm the finding that H-2f genotype is a silver-resistant H-2 genotype and that in susceptible mice, ANolA responses induced by silver is weaker than those, which are induced by mercury.
Evaluation of mercury- and silver-induced ANolA production by immunoblotting
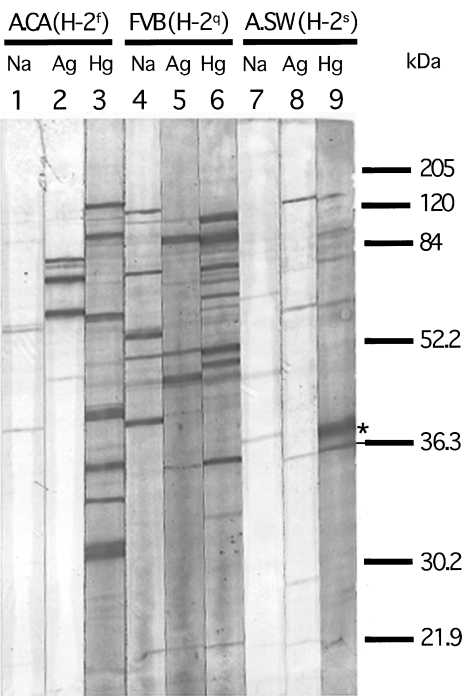
We next performed experiments to confirm that the antibody response against nucleolar antigens observed in the responder mice includes fibrillarin, the main target antigen for both mercury- and silver-induced ANolAs. Sera from mercury-, silver and saline-treated A.SW (H-2s), FVB/N (H-2q) and A.CA (H-2f) mice were analysed for their specificities against nucleolar proteins by using an immunoblotting technique. As shown in Fig. 4, sera from all mercury-treated strains [(A.SW (H-2s), FVB/N (H-2q) and A.CA (H-2f)] strongly blotted a 36-kDa nucleolar protein, corresponding to human fibrillarin [27]. Compatible with our findings with IIF, we found that only sera from silver-treated A.SW (H-2s), FVB/N (H-2q), but not A.CA (H-2f) mice were able to blot the 36-kDa protein (Fig. 4). In addition, sera from silver-treated mice blotted the fibrillarin less than mercury-injected sera (Fig. 4). These findings demonstrate that resistance to silver-induced ANolA in H-2f mice includes AFA as well.
Fig 4.
Immunoblotting of sera from mercury- and silver-treated A.CA (H-2f), FVB/N (H-2q) and A.SW (H-2s) mice using nuclear extract from Hep-2 cells. Nitrocellulose strips blotted with a Hep-2 nuclear extract were incubated with a representative serum (at 1 : 100 dilution) from an A.CA (H-2f), a FVB/N (H-2q) and an A.SW (H-2s) mouse injected with saline (lanes 1, 4 and 7), silver (lanes 2, 5 and 8) and mercury (lanes 3, 6 and 9) as described in Fig. 3. Thereafter, the bound IgG antibodies were detected with alkaline phosphatase-conjugated goat antimouse IgG1 (see for further description). A relatively strong staining of a 36-kDa band corresponding to fibrillarin (as indicated by an underlined asterisk) can be observed in all mercury-treated mice (lanes 3, 6 and 9). Sera from silver-treated FVB/N (H-2q) and A.SW (H-2s) mice also show a weak staining of the same band (lanes 5 and 8). However, the serum from the silver-treated mouse A.CA (H-2f) shows no staining of the corresponding band (lane 2).
Unresponsiveness to silver-induced ANolA in mice with H-2f genotype is independent of silver dosage and treatment duration
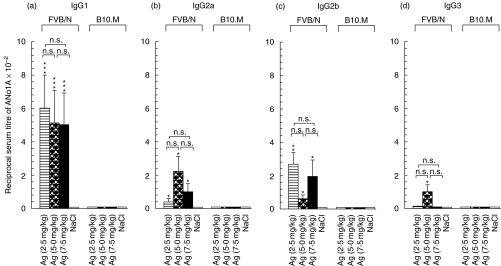
It has been shown that an optimal concentration of silver (0·01% of AgNO3 in drinking water) was required in order to induce an ANolA response in the susceptible SJL (H-2s) mice and that the development of silver-induced ANolA was proportional to duration of silver-treatment, i.e. increasing the time period for the silver-treatment resulted in the occurrence of higher serum titres of ANolA [20]. Therefore, it was possible that either the concentration of and/or treatment-duration with silver did not reach the threshold for the induction of ANolA production in H-2f mice. To test this possibility, three groups of B10.M (H-2f) and FVB/N (H-2q) mice received different subtoxic concentrations of AgNO3 (2·5 mg/kg, 5·0 mg/kg and 7·5 mg/kg body weight) for 8 (B10.M mice) or 4 (FVB/N) weeks, controls received sterile saline. At the end of the experiment, the sera were tested for the presence of ANolA of different IgG isotypes by using an IIF technique. As depicted in Fig. 5a, neither increment of silver dosage nor prolongation of silver-treatment period (from 4 weeks to 8 weeks) was able to induce ANolA production in B10.M (H-2f) mice. In FVB/N (H-2q) mice, increasing the dosage of silver to 5 mg/kg body weight caused slight increases in serum titres of IgG2a and IgG3 ANolA compared with those which were induced by 2·5 mg/kg body weight (Fig. 5b,d). However, these increases were not statistically significant.
Fig 5.
Dose–response of FVB/N (H-2q) and B10.M (H-2f) mice to silver-induced anti nucleolar autoantibody (ANolA) production. Groups of female FVB/N (H-2q) and B10.M (H-2f) mice were injected repeatedly subcutaneously (s.c.) with the indicated dosage of AgNO3 (horizontally striped, cross-hatched and solid bars) or NaCl (open bars) for either 4 (FVB/N mice) or 8 (B10.M mice) weeks. At the end of each experiment the mice were bled and killed. The sera were tested for the presence of ANolA by using an indirect immunofluorescence (IIF) method. Rat liver sections were used as the substrate. Data are shown as mean + 1 s.e. Significant differences between the parameters in silver- and saline-injected mice were calculated by Wilcoxon–Mann–Whitney test. *P < 0·05; **P < 0·01; ***P < 0·001. Significant differences between the parameters in mice injected with different concentrations of silver were calculated by multiple comparisons test. n.s.: Not significant.
F1 hybrid crosses between a silver-resistant (H-2f) and -susceptible (H-2s) genotype are resistant to silver, but not to mercury
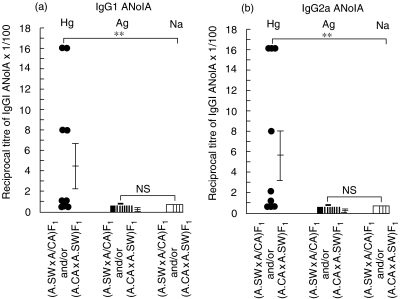
In order to investigate how unresponsiveness to silver-induced ANolA production in mice with H-2f genotype is inherited, groups of F1 hybrid crosses between silver susceptible, A.SW (H-2s) and its H-2 congenic silver resistant A.CA (H-2f) mice [(A.SW × A.CA) F1 and/or (A.CA × A.SW) F1 hybrids] were treated continuously with mercury, silver or as controls, with saline for 4 weeks. Thereafter, the development of IgG1 and IgG2a ANolA production was evaluated in these mice, using IIF method. As shown in Fig. 6a, all [9/9] the F1 hybrids (irrespective of having silver-susceptible, A.SW or -resistant, A.CA) mice as the female parental strains) responded to mercury by producing IgG1 and IgG2a ANolA. However, the magnitude of mercury-induced IgG1 and IgG2a ANolA synthesis in the F1 hybrids (Fig. 6a) was lower than those in their parental strains A.SW and A.CA (Fig. 3a).
Fig 6.
F1 hybrid crossetween a silver-resistant (H-2f) and -susceptible (H-2s) genotype are resistant to silver, but not to mercury. Groups of F1 hybrid crosses between silver-susceptible, A.SW (H-2s) and its H-2 congenic silver-resistant A.CA (H-2f) mice [(A.SW × A.CA) F1 and/or (A.CA × A.SW) F1 hybrids] were treated continuously with mercury (filled circles), silver (filled squares) or as controls, with saline (open squares) for 4 weeks. Thereafter, the mice were bled and killed. The sera were tested for the presence of IgG1 (a) and IgG2a (b) ANolA by using an indirect immunofluorescence (IIF) method. Hep-2 cell line was used as the substrate. Data are shown as mean + 1 s.e. Significant differences between the parameters in mercury-, silver- and saline-injected mice were calculated by Wilcoxon–Mann–Whitney test. **P < 0·01. n.s.: Not significant.
In contrast to mercury-injected mice, the vast majority [8/9] of silver-treated F1 hybrids exhibited no detectable titres of IgG1 and/or IgG2a ANolA in their sera (Fig. 6a). The only silver responder F1 hybrid showed a very low serum level of IgG1 and/or IgG2a ANolA (Fig. 6a). These observations suggest that resistance to silver in H-2f mice is inherited as a dominant trait in the F1 hybrids.
Discussion
In the present study, we considered the ‘cryptic peptide hypothesis’ as the working hypothesis for the heavy metal-induced ANolA production and performed experiments to address the question whether mercury and silver induce comparable ANolA responses in H-2 susceptible mouse strains. Consistent with the previous study [21], we found that similar to mercury, silver was able to induce ANolA/AFA in mice with H-2s and H2q mice, irrespective of their non-H-2 genes. These findings indicate that H-2s and H-2q genotypes per se are able to confer susceptibility to both mercury- and silver-induced ANolA/AFA production. The findings that the magnitudes of silver- and mercury-induced ANolA synthesis in B10.S (H-2s) and B10.G (H-2q) mice were lower than those in A.SW (H-2s) and FVB/N (H-2q) mice support the suggestion that non-H-2 genes in B10 mice confer a down-regulatory effect on the synthesis of ANolA induced by mercury and silver [13, 15, 16, 18, 21].
We also observed that after treatment with mercury and/or silver, susceptible mice with B10 background produced ANolA mainly of IgG2a and IgG2b isotypes, whereas mice with A or FVB/N background developed preferentially IgG1 and IgG2a ANolA. This suggests that non-H-2 genes e.g. cytokine genes that control the in vivo immunoglobulin selection [28] may also play an important role in selective appearance of IgG isotypes of metal-induced ANolAs. Therefore, it is likely that in mice with B10 background, treatment with mercury and silver predominantly activate cytokine genes that belong to CD4+ T helper type 1 cells, while in mice with A and FVB/N background similar treatments turn on both T helper type 1 and 2 cytokine genes. This likelihood is supported by our previous study, in which we demonstrated that mercury was able to induce both T helper 1 and T helper 2-types of responses in susceptible mice [29].
Although silver and mercury exhibited similarities in induction of ANolA production in susceptible H-2s and H-2q mice, there were three major differences between these xenobiotic metals with regard to ANolA synthesis. First, in the all tested susceptible strain, the magnitudes of silver-induced ANolA responses were lower than those which were induced by mercury. This indicates that silver is less potent than mercury in stimulation of the ANolA response in susceptible H-2s and H-2q mice.
The second and striking difference between the two xenobiotics was that mercury, but not silver was able to induce ANolA/AFA production in susceptible mouse strains of H-2f (B10.M and A.CA) genotype as determined by both IIF and immunoblotting. Unresponsiveness to silver-induced ANolA production in H-2f mice could not be attributed to suppressive effects of non-H-2 genes, as A.CA (H-2f) mice (with the non-H-2 genes, highly permissive for ANolA production) were completely resistant to silver. In addition, neither low dosage nor short time period of silver treatment could be accounted for the resistance to silver in H-2f mice. It has been suggested that expression of I-E molecules of MHC class II in xenobiotic-susceptible mice might have suppressive effects on the development of ANolA production [14]. However, this suggestion is not valid for silver-treated H-2f mice, as these mice do not express the I-E complex because of predominant synthesis of an Eα mRNA of aberrant size [30]. These findings indicate that H-2f mice, despite carrying susceptible H-2 genotype and having the capacity to develop ANolA, do not produce ANolA in response to silver.
The third and perhaps most striking difference between mercury and silver was that F1 hybrid crosses between H-2 congenic silver-susceptible A.SW (H-2s) and silver-resistant A.CA (H-2f) mice were found to be resistant to silver, but not mercury. This observation was novel and surprising. As silver-susceptible (H-2s) and -resistant (H-2f) H-2 haplotypes are expressed co-dominantly in the F1 hybrids, we expected to observe at least some degrees of ANolA responses in the silver-injected F1 hybrids (H-2s/f). Unresponsiveness of F1 hybrids (H-2s/f) to silver cannot be attributed to the absence of nucleolar/fibrillarin specific T cells or lack of specific T cell help, because both H-2s and H-2f haplotypes are permissive haplotypes for the development of nucleolar/fibrillarin-specific T cells. In fact, our finding that mercury could induce ANolA synthesis in the F1(H-2s/f) hybrids indicates that T cells capable of helping or activating antinucleolar/fibrillarin B cells are present in these hybrids. Thus, it seems very likely that the expression of silver-resistant H-2f haplotype (here I-Af) on the antigen presenting cells per se confers the resistance property to silver-induced ANolA synthesis in the F1 hybrids.
How do mercury- and silver-induced differential ANolA responses in H-2s, H-2q and H-2f mice and their F1 hybrids (A.SW × A.CA) F1(H-2s/f) tally with the current hypothesis ‘formation cryptic peptides’ [2,22] as the mechanism of metal-induced ANolA production? Based on our current knowledge about the presentation of cryptic self-determinants to T cells, and based on our findings, we answer this question by suggesting that mercury and silver might use similar pathways to induce ANolA synthesis. However, they create and/or display dissimilar self cryptic-peptides, which lead to differential production of ANolA in the susceptible strains. Two types of experimental and hypothetical evidence can support our suggestion. First, the results from studies in which mouse lysozyme (ML) was used as a model of self-protein, have shown that there was a strong association between the crypticity of self-determinants and the H-2 of the host and that a cryptic determinant in one H-2 haplotype was not necessarily cryptic in another one [31]. If we apply these statements in the context of our above-mentioned findings, we can conceive that mercury and silver by having different affinity for organic donors (owing to their chemical properties) form dissimilar complexes with the nucleolar protein, fibrillarin. While mercury–fibrillarin complexes would lead to the creation and display of determinants, which are cryptic in the context of H-2s, H-2q and H-2f genotypes, silver–fibrillarin complexes would create determinants that are cryptic only in the context of H-2s and H-2q, but not H-2f genotype.
Secondly, our finding that H-2f mice and their F1(H-2s/f) hybrids were resistant to silver-, but not mercury-induced ANolA production can also be explained by the determinant capture hypothesis [32]. This hypothesis suggests that during antigen processing, the unfolding antigen binds to an MHC through its most available determinant and is then trimmed down to the final size, while the rest of the antigen molecule, including cryptic determinants is discarded [32]. Moreover, according to this hypothesis, when there are several MHC molecules on the APCs, there will be a competition among these different MHC molecules competing for binding to the same processed determinant. Therefore, it is possible that in silver-injected H-2f mice and their F1(H-2s/f) hybrids, during the unfolding of fibrillarin, I-Af molecules bind effectively to dominant or subdominant fibrillarin determinants and discard the cryptic-self determinants. Thus, the lack of crypticity and/or the absence of cryptic determinants would result in unresponsiveness to silver-induced ANolA production in mouse strains with H-2f genotype as well as in their F1(H-2s/f) hybrids.
Although our findings could be explained in the context of ‘formation cryptic self-peptides’, they do not dismiss the possibility that merely the creation of cryptic self-peptide by a xenobiotic is insufficient for the induction of ANolA responses. In fact, the current model of T cell activation enlightens this possibility. With regard to this model, T cell activation requires two signals, a specific one, which is acquired by the interaction between specific T cell receptor and MHC/peptide presented by an antigen-presenting cell (APC) and a non-specific one, which is provided under inflammatory conditions and results in the expression of co-stimulatory molecules such as B7 [33]. By applying this model to the xenobiotic metal-induced ANolA production, one can conceive that merely the displaying of cryptic self-peptides (signal one) by a metal would not be sufficient for induction of an ANolA response and that the expression of co-stimulatory molecules is also required. Therefore it is likely that, compared to mercury, silver is generally less efficient in providing co-stimulating conditions (as it is less toxic than mercury) for ANolA production in H-2 susceptible strains, i.e. the lowest efficacy can be seen in the mice with H-2f genotype. In fact, our finding that compared to mercury-injected mice, silver-injected mice exhibited lower magnitudes of ANolA responses in all tested strains is in agreement with this likelihood.
Taken together, the findings in our study have important implications in understanding the susceptibility or resistance to some autoimmune diseases such as rheumatoid arthritis in which genetic factors (particularly MHC class II genes) and environmental factors are believed to play important roles in predisposition and development of the disease [34]. Our results also suggest that a particular autoimmune-associated susceptible MHC gene or locus, in combination with another susceptible and/or resistant MHC allele, might significantly change the susceptibility pattern towards the protection from the disease.
Acknowledgments
We thank professor Klavs. Berzins and Dr Evren Alici for reading the manuscript. This study was supported by a grant from the Swedish Foundation for Health Care Sciences and Allergy Research.
References
- 1.Goldman M, Druet P, Gleichmann E. Th2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–7. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 2.Griem P, Gleichmann E. Metal ion induced autoimmunity. Curr Opin Immunol. 1995;7:831–8. doi: 10.1016/0952-7915(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 3.Eneström S, Hultman P. Does amalgam affect the immune system? A controversial issue. Int Arch Allergy Immunol. 1995;106:180–203. doi: 10.1159/000236843. [DOI] [PubMed] [Google Scholar]
- 4.Goter Robinson CJ, Abraham Balazs T. Induction of anti-nuclear antibodies by mercuric chloride in mice. Clin Exp Immunol. 1984;58:300–6. [PMC free article] [PubMed] [Google Scholar]
- 5.Hultman P, Eneström S. Mercury induced B-cell activation and antinuclear antibodies in mice. Clin Lab Immunol. 1989;28:143–50. [PubMed] [Google Scholar]
- 6.Hultman P, Eneström S, Pollard KM, Tan EM. Anti-fibrillarin autoantibodies in mercury-treated mice. Clin Exp Immunol. 1989;78:470–2. [PMC free article] [PubMed] [Google Scholar]
- 7.Reuter R, Tessars G, Vohr H-W, Gleichmann E, Luhrmann R. Mecuric chloride induces autoantibodies against U3 small nuclear ribonucleoprotein in susceptible mice. Proc Natl Acad Sci USA. 1989;86:237–41. doi: 10.1073/pnas.86.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi K, Turley SJ, Tan EM, Pollard KM. Analysis of the autoantibody response to fibrillarin in human disease and murine models of autoimmunity. J Immunol. 1995;154:961–71. [PubMed] [Google Scholar]
- 9.Fournier MJ, Maxwell ES. The nucleolar snRNAs: catching up with the spliceosomal snRNAs. Trends Biochem Sci. 1993;18:131–5. doi: 10.1016/0968-0004(93)90020-n. [DOI] [PubMed] [Google Scholar]
- 10.Hultman P, Johansson U, Dagnaes-Hansen F. Murine mercury-induced autoimmunity. the role of T-helper cells. J Autoimmun. 1995;8:809–23. doi: 10.1016/s0896-8411(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 11.Kasturi KN, Hatakeyama A, Spiera H, Bona CA. Antifibrillarin autoantibodies present in systemic sclerosis and other connective tissue diseases interact with similar epitopes. J Exp Med. 1995;181:1027–36. doi: 10.1084/jem.181.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnett FC, Reveille JD, Goldstein R, et al. Autoantibodies to fibrillarin in systemic sclerosis (scleroderma). An immunogenetic, serologic, and clinical analysis. Arthritis Rheum. 1996;39:1151–60. doi: 10.1002/art.1780390712. [DOI] [PubMed] [Google Scholar]
- 13.Goter Robinson JC, Balazs T, Egorov IK. Mercuric chloride-, gold sodium thiomalate-, and d-penicillamine-induced antinuclear antibodies in mice. Toxicol Appl Pharmacol. 1986;86:159–69. doi: 10.1016/0041-008x(86)90046-3. [DOI] [PubMed] [Google Scholar]
- 14.Mirtcheva J, Pfeiffer C, De Bruijn JA, Jacquesmart F, Gleichmann E. Immunological alterations inducible by mercury compounds. III. H-2A acts as an immune response and H-2E as an immune ‘suppression’ locus for HgCl2-induced antinucleolar autoantibodies. Eur J Immunol. 1989;19:2257–61. doi: 10.1002/eji.1830191212. [DOI] [PubMed] [Google Scholar]
- 15.Hultman P, Bell LJ, Eneström S, Pollard KM. Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic and intra-H-2 recombinant strains. Clin Immunol Immunopathol. 1992;65:98–109. doi: 10.1016/0090-1229(92)90212-7. [DOI] [PubMed] [Google Scholar]
- 16.Hultman P, Bell LJ, Eneström S, Pollard KM. Murine susceptibility to mercury. II. Autoantibody profiles and renal immune deposits in hybrid, backcross, and H-2d congenic mice. Clin Immunol Immunopathol. 1993;68:9–20. doi: 10.1006/clin.1993.1088. [DOI] [PubMed] [Google Scholar]
- 17.Hultman P, Turley SJ, Eneström S, Lindh U, Pollard KM. Murine genotype influences the specificity, magnitude and persistence of murine mercury-induced autoimmunity. J Autoimm. 1996;9:139–49. doi: 10.1006/jaut.1996.0017. [DOI] [PubMed] [Google Scholar]
- 18.Abedi-Valugerdi M, Möller G. Contribution of H-2 and non-H-2 genes in the control of mercury-induced autoimmunity. Int Immunol. 2000;12:1425–30. doi: 10.1093/intimm/12.10.1425. [DOI] [PubMed] [Google Scholar]
- 19.Abedi-Valugerdi M, Hansson M, Möller G. Genetic control of resistance to mercury-induced immune/autoimmune activation. Scand J Immunol. 2001;54:190–7. doi: 10.1046/j.1365-3083.2001.00932.x. [DOI] [PubMed] [Google Scholar]
- 20.Hultman P, Eneström S, Turley SJ, Pollard KM. Selective induction of anti-fibrillarin autoantibodies by silver nitrate in mice. Clin Exp Immunol. 1994;96:285–91. doi: 10.1111/j.1365-2249.1994.tb06555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hultman P, Ganowiak K, Turley SJ, Pollard KM. Genetic susceptibility to silver-induced anti-fibrillarin autoantibodies in mice. Clin Immunol Immunopathol. 1995;77:291–7. doi: 10.1006/clin.1995.1155. [DOI] [PubMed] [Google Scholar]
- 22.Johansson U, Hansson-Georgiadis H, Hultman P. Murine silver-induced autoimmunity: silver shares induction of antinucleolar antibodies with mercury, but causes less activation of the immune system. Int Arch Allergy Immunol. 1997;113:432–43. doi: 10.1159/000237619. [DOI] [PubMed] [Google Scholar]
- 23.Frausto da Silva JJR, Williams RJP. The biological chemistry of the elements: the inorganic chemistry of life. 1. New York: Oxford University Press; 1991. [Google Scholar]
- 24.Behrens S-E, Kastner B, Luhrman R. Preparation of U small nuclear ribonucleoprotein particles. In: Celis JE, editor. Cell biology: a laboratory handbook. Vol. 1. San Diego, CA: Academic Press; 1994. pp. 628–41. [Google Scholar]
- 25.Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aris JP, Blobel G. cDNA cloning and sequencing of human fibrillarin, a conserved nucleolar protein recognized by autoimmune antisera. Proc Natl Acad Sci USA. 1991;88:931–5. doi: 10.1073/pnas.88.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–35. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 29.Hu H, Möller G, Abedi-Valugerdi G. Mechanism of mercury-induced autoimmunity: both T helper 1- and T helper 2-type responses are involved. Immunol. 1999;96:348–57. doi: 10.1046/j.1365-2567.1999.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathis DJ, Benoist C, Virginia E, Williams IIVE, Kanter M, McDevitt HO. Several mechanisms can account for defective Eα gene expression in different mouse haplotypes. Proc Natl Acad Sci USA. 1983;80:273–7. doi: 10.1073/pnas.80.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moudgil KD, Sercarz EE. Dominant determinants in hen eggwhite lysozyme correspond to the cryptic determinants within its self-homologue, mouse lysozyme: implications in shaping of the T cell repertoire and autoimmunity. J Exp Med. 1993;178:2131–8. doi: 10.1084/jem.178.6.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–66. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 33.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 34.Andersson EC, Svendsen P, Svejgaard A, Holmdahl R, Fugger L. A molecule basis for the HLA association in rheumatoid arthritis. Rev Immunogenet. 2000;2:81–7. [PubMed] [Google Scholar]