Abstract
The aim of our study was to estimate the populations of peripheral blood myeloid and lymphoid dendritic cells (CD1c+, BDCA-2+) and the CD1c+ : BDCA-2+ ratio in normal pregnant women and in patients with pre-eclampsia. Fifteen women in the first, second and third trimesters of normal pregnancy, and 25 patients with pre-eclampsia were included in the study. The dendritic cells were isolated from peripheral blood, stained with monoclonal antibodies against blood dendritic cell antigens (anti-CD1c, anti-BDCA-2) and estimated using the flow cytometric method. CD1c+ and BDCA-2+ dendritic cells were present in women during all trimesters of physiological pregnancy and in pre-eclamptic patients. It was observed that the numbers of dendritic cells were significantly lower in the second trimester when compared with the first and third trimesters of normal pregnancy. Furthermore, in the second trimester, CD1c+ : BDCA-2+ ratio was higher than in the other trimesters of physiological pregnancy. All populations of dendritic cells and CD1c+ : BDCA-2+ ratio did not differ in the first and third trimesters of normal pregnancy. The percentage of BDCA-2+ dendritic cells was significantly lower in pre-eclampsia in comparison with healthy women in the third trimester of physiological pregnancy, while CD1c+ : BDCA-2+ ratio was significantly higher in pre-eclamptic patients when compared with control groups. We concluded that dendritic cells may be involved in the immune regulation during physiological pregnancy. CD1c+ and BDCA-2+ cells can influence the Th2 phenomenon which is observed during physiological pregnancy. Furthermore, it seems possible that lower BDCA-2+ cells percentage and higher CD1c+ : BDCA-2+ ratio can be associated with increased Th1-type immunity in patients with pre-eclampsia.
Keywords: dendritic cells, pre-eclampsia, pregnancy
INTRODUCTION
Pre-eclampsia is a common obstetric syndrome affecting about 7–10% of pregnant women. The symptoms of this syndrome appear during the second and third trimesters of pregnancy. It is known that pre-eclampsia is a placental disorder developing in two stages [1,2]. The first consists in poor transformation of the spiral arteries and the impaired cytotrophobast differentiation. Pre-eclampsia is characterized by shallow cytotrophoblast invasion and decreased blood flow to the placenta. It has been shown lately that invasive cytotrophoblast fails to properly modulate their integrin expressions in pre-eclampsia [3–5]. Furthermore, widespread apoptosis of placental cytotrophoblasts within the uterine wall has been observed in pre-eclampsia [6,7]. The second stage of the disease comprises the consequences of placental ischaemia.
It seems that abnormal activation of the immune system may play a role in the aetiology of pre-eclampsia. Many authors have found a number of changes in the adaptive immune system which may contribute to the development of pre-eclampsia [8–10]. Recent data suggest that pre-eclampsia is a T-helper 1/T-helper 2 immunity disorder with predominant Th1 type immunity [9,10]. Furthermore, there is evidence regarding the activation of the innate immune system in pre-eclampsia [11]. It has been shown recently that normal third trimester pregnancy is characterized by the activation of peripheral blood leucocytes, which is further increased in pre-eclampsia [11]. Interestingly, the changes in innate immunity can influence T-helper 1/T-helper 2 cytokine balance; for example, endotoxin administration can cause the shift of T-cell immunity response towards Th2 cell type response [12].
Dendritic cells (DCs) are antigen-presenting cells with an ability to induce primary immune responses. They capture and transfer information from the outside world to the cells of the adaptive immune system. DCs are not only critical for the induction of primary immune responses, but may also be important for the induction of immunological tolerance, as well as for the regulation of the type of T-cell mediated immune responses. Two distinct lineages of DCs have been described in humans. Myeloid dendritic cells are a major subpopulation of human blood dendritic cells which are CD4+, Lin−, CD11cbright, CD123dim, CD45RO+ and CD2+. They express myeloid markers (CD13, CD33) as well as Fc receptors (CD32, CD64, Fc∈RI) and are of monocytoid morphology. Myeloid dendritic cells (DC-1) also have the expression of BDCA-2 (CD1c) antigen which is characteristic of peripheral blood DC-1 cells. Plasmocytoid (lymphoid) dendritic cells (DC-2) have been recently described in human peripheral blood and lymphoid tissue. Peripheral blood DC-2 cells express a specific BDCA-2 marker. Phenotyping of BDCA-2+ dendritic cells in peripheral blood characterizes these cells as being CD4+, Lin−, CD11c−, CD123bright, CD45RA+, CD2− and expressing neither myeloid lineage markers like CD13 and CD33, nor Fc receptors like CD32, CD64 or Fc∈RI. After appropriate activation, DC-2 cells induce T-cell differentiation into Th2 cells [13–15].
The aim of our study was to investigate the populations of myeloid DC-1 dendritic cells (CD1c+) and lymphoid DC-2 dendritic cells (BDCA-2+) and CD1c+ : BDCA-2+ ratio in peripheral blood of pregnant women in the first, second and third trimesters of physiological pregnancy, and in patients with pre-eclampsia.
MATERIALS AND METHODS
Patients
The patients participating in the study were admitted to the Department of Obstetrics and Perinatology of the University School of Medicine in Lublin. The study group comprised 25 patients with pre-eclampsia at 34–40 weeks of gestation. Patients with pre-eclampsia were admitted to the hospital because of the symptoms of the disease and not because of signs of labour. Fifteen healthy women with uncomplicated pregnancies were the control group. We have taken blood samples from 15 women throughout their pregnancies (in the first, second and third trimesters) to minimize patient variance. The gestational age was similar in each control group. The gestational age of women in the third trimester of normal pregnancy was matched to the pre-eclamptic group. Patients in the third trimester were not in active labour. The study design was accepted by the local Ethics Committee. Informed consent from the patients for peripheral blood sampling was obtained. Pre-eclamptic patients were classified according to the International Society for the Study of Hypertension in Pregnancy [16]. Pre-eclampsia was characterized on the basis of the following symptoms: blood pressure of at least 140/90 mmHg and proteinuria above 0·3 g/24 h. None of the pre-eclamptic patients was affected by pre-existing clinical disorders such as chronic hypertension or renal diseases before pregnancy, and none of the pregnancies was complicated by preterm labour or chorioamnionitis. All pregnancies in the study and control groups were single.
Blood sampling and cell preparation
Blood samples from pre-eclamptic patients were taken at the moment of admission to the hospital. Blood (20 ml) was taken by venipuncture from each patient and each woman from the control group and collected in sterile heparinized tubes.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were separated by gradient centrifugation on the lymphocyte separation medium, Lymphoprep (Nycomed, Torshov, Norway). They were centrifuged for 30 min at 250 g at 4°C, collected from the interface with a Pasteur pipette and washed twice by centrifugation for 5 min at 250 g at 4°C in the buffer containing phosphate buffered saline (PBS, Serum and Vaccine Factory, Biomed, Lublin, Poland) without Ca2+ and Mg2+, with 0·5% bovine serum albumin (BSA, Sigma, St Louis, MO, USA) and 2 mm EDTA (Sigma).
Phenotyping of cells
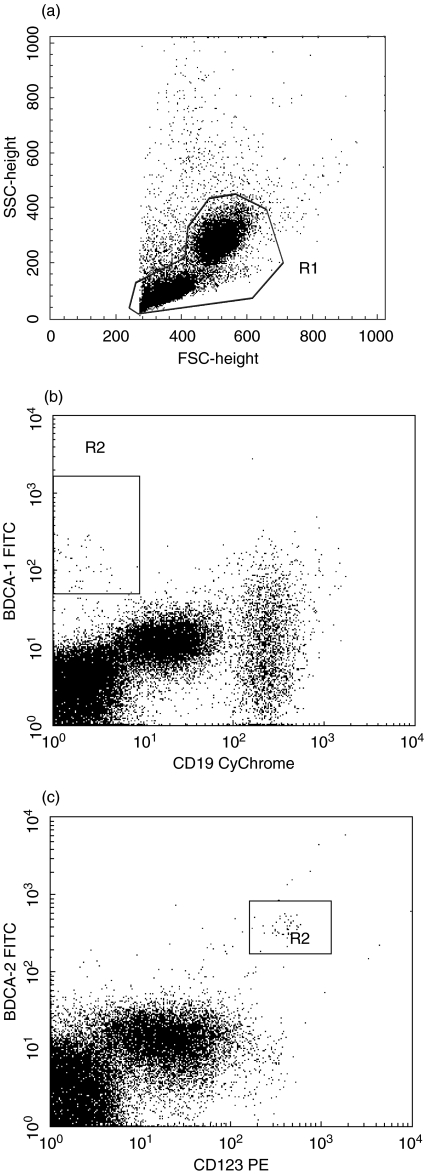
The cell surface antigens in each case were determined on the fresh cells at the time of the sample submission. The cells were labelled by direct staining with monoclonal antibodies. The following directly conjugated monoclonal antibodies (mAbs) were used: mouse anti-human CD1c-FITC (Miltenyi-Biotec, Bergisch Gladbach Germany), BDCA-2-FITC (Miltenyi-Biotec), CD123-PE (Becton Dickinson, Franklin Lakes, NJ, USA), CD19-CyChrome (Pharmingen, San Diego, CA, USA). Mouse anti-human IgG2a isotype control was used for anti-CD1c staining. Mouse anti-human IgG1 isotype control was used for anti BDCA-2 staining. After washing, 20 µl of FcR Blocking Reagent (Miltenyi-Biotec) were added to every 107 cells resuspended in 80 µl of the buffer. Monoclonal antibodies were added according to the manufacturer's protocol and incubated with the cells for 10 min in the dark at 4°C. After incubation, the cells were washed in 10 volumes of the buffer and centrifuged for 10 min at 300 g and 4°C. Next, the cells were collected using a FACSCalibur flow cytometer equipped with 488-nm argon laser (Becton Dickinson) and analysed with CellQuest Software. We collected 300 000 of events in total. Cell debris and dead cells were excluded from the analysis based on scatter signals. CD1c marker is expressed on a subpopulation of CD19+ small resting B-lymphocytes. The mononuclear cell analysis region was analysed for CD1c and CD19 staining. CD1c+ B cells were excluded from CD1c+ blood dendritic cells by counter-staining for CD19. CD1c+ CD19− cells were counted as circulating myeloid DCs. Next, the mononuclear cell analysis region was analysed for BDCA-2 and CD123 antigens. BDCA-2+CD123+ cells are counted as circulating lymphoid DCs. The identification of circulating DCs by flow cytometry in peripheral blood is presented in Fig. 1.
Fig. 1.
The identification of circulating DCs by flow cytometry in peripheral blood of one patient with pre-eclampsia. (a) The mononuclear cell analysis region (R1) applied to light scatters. (b) The R1-gated events were analysed for BDCA-1 and CD19 staining and BDCA-1+CD19− cells were counted as circulating myeloid DCs. (c) The R1-gated events were then analysed for BDCA-2 and CD 123 and BDCA-2+CD123+ cells were counted as circulating lymphoid DCs.
Statistical analysis
Statistical differences between groups were estimated using a standard non-parametric test (Mann–Whitney U-test). The results were presented as median with the interquartile ranges. Differences at P < 0·05 were considered as statistically significant. Commercially available software Statistica 5·0 PL was applied to statistical analysis.
RESULTS
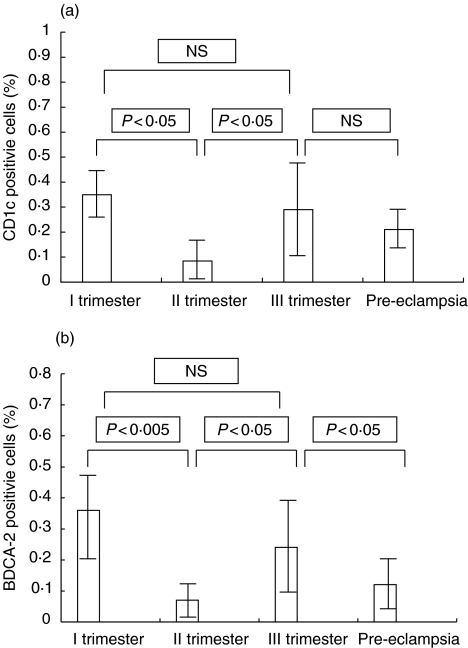
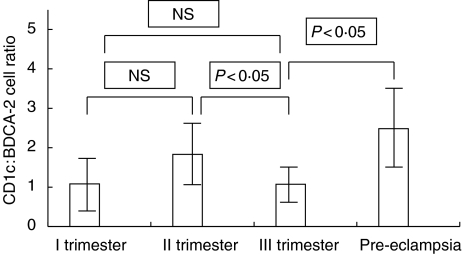
CD1c+ and BDCA-2+ cells were present in women during all trimesters of normal pregnancy and in pre-eclamptic patients. The absolute numbers of myeloid and lymphoid dendritic cells in the study and control groups are presented in Table 1. It was observed that the percentages of dendritic cells were significantly lower in the second trimester when compared with the first and third trimesters of pregnancy (II trimester – CD1c+: median, 0·085%, interquartile ranges, 0·05% to 0·2%; BDCA-2+: median, 0·07%, interquartile ranges, 0·03% to 0·115%; I trimester – CD1c+: median, 0·35%, interquartile ranges, 0·235 to 0·44%; BDCA-2+: median, 0·36%, interquartile ranges, 0·145% to 0·485%; III trimester – CD1c+: median, 0·29%, interquartile ranges, 0·1% to 0·45%; BDCA-2+: median, 0·24%, interquartile ranges, 0·11% to 0·37%). The results are presented in Fig. 2(a and b). Furthermore, in the second trimester, CD1c+ : BDCA-2+ ratio was higher than in the other trimesters of normal pregnancy (II trimester CD1c+ : BDCA-2+ ratio – median, 1·83, interquartile ranges, 1·237 to 2·416; I trimester CD1c+ : BDCA-2+ ratio – median, 1·083, interquartile ranges, 0·753 to 1·917; III trimester CD1c+ : BDCA-2+ ratio – median, 1·074, interquartile ranges, 0·97 to 1·454) but when compared with the first trimester of pregnancy the differences were not statistically significant. The results are presented in Fig. 3. The population of CD1c+ cells did not differ in the group of pre-eclamptic patients when compared with the third trimester of normal pregnancy (CD1c+: median, 0·21%, interquartile ranges, 0·14% to 0·305 vs. 0·29%, interquartile ranges, 0·1% to 0·45%; NS, not statistically significant). The percentage of BDCA-2+ cells was significantly lower in pre-eclampsia in comparison with healthy women in the third trimester of pregnancy, while CD1c+ : BDCA-2+ ratio was significantly higher in pre-eclamptic patients when compared with the control group in III trimester (BDCA-2+: median, 0·12%, interquartile ranges, 0·055% to 0·215% vs. 0·24%, interquartile ranges, 0·11% to 0·37%; P < 0·05; CD1c+ : BDCA-2+ ratio – 2·48, interquartile ranges, 1·229 to 3·0 vs. 1·074, interquartile ranges, 0·97 to 1·454, P < 0·005). The results are presented in Fig. 2(a,b) and Fig. 3.
Table 1.
The absolute numbers of CD1c+ and BDCA-2+ cells (cells/mm3 of blood) in the group of patients with pre-eclampsia (n = 25) and in normal pregnant women (n = 15)
| I trimester median (interquartile ranges) | II trimester median (interquartile ranges) | III trimester median interquartile ranges) | Pre-eclampsia median (interquartile ranges) | |
|---|---|---|---|---|
| CD1c+ | 520 (261–1152) | 367 (176–501)* | 656 (267–1103)* | 600 (401–791) |
| BDCA-2+ | 420 (256–1262) | 183 (100–272)** | 617 (292–933)*** | 365 (128–527)* |
n, number of patients;
P < 0·05, BDCA-2+ cells between pre-eclamptic group and third trimester group; CD1c+ cells between first and second trimester groups; CD1c+ cells between second and third trimester groups;
P < 0·01, BDCA-2+ cells between first and second trimester groups;
P < 0·005, BDCA-2+ cells between second and third trimester groups.
Fig. 2.
(a) The percentages of BDCA-1+ cells in pre-eclampsia (n = 25) and in the first, second and third trimesters of physiological pregnancy (n = 15). (b) The percentages of BDCA-2+ cells in pre-eclampsia (n = 25) and in the first, second and third trimesters of physiological pregnancy (n = 15).
Fig. 3.
The BDCA-1+ : BDCA-2+ ratio in pre-eclampsia (n = 25) and in the first, second and third trimesters of physiological pregnancy (n = 15).
DISCUSSION
There are many changes in the immunological state during physiological pregnancy. According to Wegmann's hypothesis, a successful pregnancy is a ‘Th2 phenomenon’[17]. Several authors observed Th1/Th2 immunity alterations with a shift to predominant Th2-type immunity during normal pregnancy [18,19]. It is not known what factors can influence the Th2 predominance in normal pregnancy. It has been shown lately that IL-4 rather than other Th1 or Th2 cytokines is produced by cytotrophoblast cells. These findings can suggest that in human pregnancy placental cytotrophoblast cells can modulate a Th2 bias [20]. Alternatively, newly discovered cytokines do not fit into the classical Th1/Th2 dichotomy. The cytokines, such as: IL-11, IL-12, IL-13, IL-15, IL-16, IL-17 and IL-18, were detected in the uterus, the peri-implantaion embryo, and in decidual and placental tissues throughout pregnancy [21]. It can suggest their important regulatory role, especially during implantation. They can be engaged in the materno-foetal relationship through the influence on the activation of NK cells, on the action of adhesion molecules and on the process of vascularization [21].
Predominant Th1-type immunity is correlated with spontaneous abortions or intrauterine growth retardation [22,23]. It was shown that spontaneous abortions in pregnant women might be associated with Th1-type immunity to trophoblast [22,23]. Furthermore, it has been recently proposed that pre-eclampsia is Th1/Th2 immunity disorder with predominant Th1-type immunity [10,11].
T-helper CD4+ lymphocytes are divided into three subsets, according to the type of produced cytokines [24,25]. T-helper 1 (Th1) lymphocytes produce interleukin-2 and IFN-γ and induce cell-mediated immunity. T-helper 2 (Th2) lymphocytes produce IL-4, IL-5, IL-10 and IL-13 and enhance B-lymphocyte proliferation and differentiation (in general humoral-mediated immunity). These subpopulations of T-helper lymphocytes originate from the third subset of T-helper lymphocytes, T-helper 0 (Th0) lymphocytes, which produce both types of cytokines [24–29].
Dendritic cells (DCs) are the only antigen-presenting cells that can prime naive T cell to a new antigen. Peripheral blood myeloid dendritic cells (CD1c+) express myeloid antigens CD11c, CD13 and CD 33, they originate from myeloid bone marrow precursors and require the presence of GM-CSF for their survival. In humans, peripheral blood CD1c+ cells are identified as negative for lymphoid and myeloid cell-specific markers (lin−) and are HLA-DR+/CD11c+. CD1c+ cells produce high levels of IL-12 when stimulated with tumour necrosis factor-α (TNF-α) or ligand for CD40 and drive T-cell differentiation into Th1 lymphocytes. Lymphoid dendritic cells (BDCA-2+) have recently been described in human peripheral blood and lymphoid tissue as HLA-DR+/lin−/CD11c−/CD4+IL-3Rα+ plasmacytoid cells. After appropriate activation, BDCA-2+ cells induce T-cell differentiation into Th2 cells [13–31].
For that reason we have made an effort to estimate the populations of myeloid (CD1c+) and lymphoid (BDCA-2+) blood dendritic cells in women during physiological pregnancy, which is called a ‘Th2 phenomenon’, and in patients with pre-eclampsia, which is thought to be Th1-type immunity disease.
It has been recently proposed that pre-eclampsia is associated with Th1/Th2 imbalance and predominant Th1-type immunity [9,10]. It has been found that serum IL-2 concentrations are elevated in women with pre-eclampsia [32]. Recent Japanese studies demonstrated that serum IL-2 and TNF-α concentrations were higher in the first trimester of pregnancy in women who later developed pre-eclampsia [33]. These findings can suggest that impairment of immune regulation occurs in early pregnancy before the clinical manifestation of pre-eclampsia. Futhermore, the expression of interleukin-2 has been found in the decidual cells of pre-eclamptic patients [34]. Many authors observed increased interleukin-12 concentrations in patients with pre-eclampsia and HELLP syndrome [35–37]. Furthermore, it has recently been observed that the secretion of IL-12 by peripheral blood mononuclear cells was increased in pre-eclampsia and decreased in normal pregnant women [38]. It seems possible that increased interleukin-12 secretion by activated monocytes can cause Th1 predominance in pre-eclampsia [38].
It is not known what are the possible regulatory mechanisms that might be involved in the alterations in the myeloid and lymphoid dendritic cells in pre-eclampsia. It seems possible that such cytokines as GM-CSF, IL-4 and TNF-α, which are the growth factors for myeloid dendritic cell maturation, are engaged in CD1c+ : BDCA-2+ shift. It has been observed recently that in patients with pre-eclampsia there are high levels of circulating TNF-α[39–42]. It is known that TNF-α is capable of inducing IL-12 in CD1c+ cells. It seems possible that TNF-α can stimulate maturation of myeloid dendritic cells and the production of IL-12 by mononuclear cells of pre-eclamptic patients. It has also been observed that high levels of IL-4 are associated with pre-eclampsia in the second half of pregancy and in puerperium [43]. However, serum levels of GM-CSF were detected less frequently and in lower concentrations in patients with pre-eclampsia when compared with healthy pregnant women [44].
IL-12 is produced by antigen-presenting cells and stimulates differentiation of T-helper 0 cells into Th1 lymphocytes. It seems possible that one of the sources of IL-12 in pre-eclampsia is myeloid dendritic cells. In our study, the numbers of myeloid cells did not differ in the group of patients with pre-eclampsia in comparison with healthy women in the third trimester of pregnancy. However, the percentages of BDCA-2+ lymphoid cells were significantly lower in the group of pre-eclamptic patients when compared with controls. Furthermore, CD1c+ : BDCA-2+ ratio was significantly higher in the group of pre-eclamptic patients in comparison with normal, pregnant women in the third trimester. It is known that lymphoid dendritic cells are responsible for stimulation of Th0 cells into Th2 lymphocytes. It seems possible that the decreased percentage of BDCA-2+ cells is associated with the impairment of Th0 into Th2 cell differentiation in the group of patients with pre-eclampsia. Furthermore, increased CD1c+ : BDCA-2+ ratio in pre-eclamptic patients can favour Th1 lymphocyte differentiation in these patients. The similar changes have been observed in rheumatoid arthritis [45]. Recently, it has been suggested that progenitors of myeloid dendritic cells exisiting in synovial fluid are participants in the rheumatoid arthritis disease process. It has been observed that mature myeloid DCs drived from rheumatoid arthritis synovial fluid progenitors were potent stimulators of allogeneic T cells and Th1-type response [45].
In our study, the percentage of CD1c+, BDCA-2+ cells and CD1c+ : BDCA-2+ ratio did not differ in the groups of women in the first and third trimesters of physiological pregnancy. However, in the second trimester of normal pregnancy we observed that the percentages of all populations of dendritic cells were significantly decreased when compared with the other trimesters of normal pregnancy. In reverse, CD1c+ : BDCA-2+ ratio was higher in healthy women in the second trimester, but, when compared with the first trimester of pregnancy, the differences were not statistically significant. It is difficult to say what the reasons for the alterations during the second trimester of physiological pregnancy are, but it seems possible that they are associated with the changes in the pregnancy hormone concentrations. It has been suggested recently that Th1- and Th2-type cytokines during pregnancy are hormone controlled [19]. It also seems possible that dendritic cells are hormone controlled during physiological pregnancy. At the turn of the first and second trimester, corpus luteum decreases the production of progesteron and placenta takes over this function. A decrease in the dendritic cells population during the second trimester of normal pregnancy can be associated with the change in the source of progesteron production and with the fluctuations of progesteron concentrations.
In conclusion, dendritic cells may be involved in the immune regulation during physiological pregnancy. CD1c+, BDCA-2+ dendritic cells and CD1c+ : BDCA-2+ ratio can influence the Th2 phenomenon which is observed during physiological pregnancy. Furthermore, it seems possible that significantly lower BDCA-2+ cells percentage and higher CD1c+ : BDCA-2+ ratio are associated with increased Th1-type immunity which is observed in patients with pre-eclampsia.
Acknowledgments
This work was supported by grant No. 3P05E 154 22 from The State Committee for Scientific Investigations.
References
- 1.Brown MA. The physiology of pre-eclampsia. Clin Exp Pharmacol Physiol. 1995;22:781–91. doi: 10.1111/j.1440-1681.1995.tb01937.x. [DOI] [PubMed] [Google Scholar]
- 2.Redman. CWG. Immunological aspects of pre-eclampsia. Baillieres Clin Obstet Gynecol. 1992;6:601–15. doi: 10.1016/s0950-3552(05)80012-4. [DOI] [PubMed] [Google Scholar]
- 3.Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, Fisher SJ. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol. 1997;151:1809–18. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is asociated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–60. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Genbacev O, Damsky CH, Fisher SJ. Oxygen regulates human cytotrophoblast differentiation and invasion: implications for endovascular invasion in normal pregnancy and in pre-eclampsia. J Reprod Immunol. 1998;39:197–213. doi: 10.1016/s0165-0378(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 6.DiFederico E, Genbacev O, Fisher SJ. Pre-eclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genbacev O, DiFederico E, McMaster M, Fisher SJ. Invasive cytotrophoblast apoptosis in pre-eclampsia. Hum Reprod. 1999;2:59–66. doi: 10.1093/humrep/14.suppl_2.59. [DOI] [PubMed] [Google Scholar]
- 8.Bettin S, Halle H, Wenzkowski BM, Volk HD, Jahn S. Immunologic parameters in women with normal pregnancy and pre-eclampsia. Zentralbl Gynakol. 1994;116:260–2. [PubMed] [Google Scholar]
- 9.Saito S, Umekage H, Sakamoto Y, Sakai M, Tanebe K, Sasaki Y, Morikawa H. Increased T Helper 1 type immunity and decreased T helper 2 type immunity in patients with pre-eclampsia. Am J Reprod Immunol. 1999;41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 10.Saito S, Sakai Y, Sasaki K, Tanebe H, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1: Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–5. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacks GP, Studena K, Sargent IL, Redman CWG. Normal pregnancy and preeclampsia both produce inflamatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 12.Zimmer S, Pollard V, Marshall GD, Garofalo RP, Traber D, Prough D, Herndon DN. The 1996 Moyer Award. Effects of endotoxin on the Th1/Th2 response in humans. J Burn Care Rehabil. 1996;17:491–6. [PubMed] [Google Scholar]
- 13.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–46. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Briere F, Caux Ch, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka P. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 15.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CDL. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–78. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Davey DA, MacGillivray I. The classification and definition of the hypertensive disorders of pregnancy. Am J Obstet Gynecol. 1988;158:892–8. doi: 10.1016/0002-9378(88)90090-7. [DOI] [PubMed] [Google Scholar]
- 17.Wegmann TG. Bi-directional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;7:353–35. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 18.Lin H, Mosmann TR, Guilbert L, Tuntipopitat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal–fetal interface. J Immunol. 1993;151:4562–73. [PubMed] [Google Scholar]
- 19.Piccinni MP, Romagnani S. Regulation of fetal allograft survival by a hormone-controlled Th1- and Th2-type cytokines. Immunol Res. 1996;15:141–50. doi: 10.1007/BF02918503. [DOI] [PubMed] [Google Scholar]
- 20.Sacks GP, Clover LM, Bainbridge DRJ, Redman CWG, Sargent IL. Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta. 2001;22:55–9. doi: 10.1053/plac.2001.0686. [DOI] [PubMed] [Google Scholar]
- 21.Chaouat G, Zourbas S, Ostojic S, Lappree-Delage G, Dubanchet S, Ledee N, Martal J. A brief review of recent data on some cytokine expressions at the materno–foetal interface which might challenge the classical Th1/Th2 dichotomy. J Reprod Immunol. 2002;53:241–5. doi: 10.1016/s0165-0378(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 22.Marzi M, Vigano A, Trabattoni D, Villa MI, Salvaggio A, Clerici E. Characterisation of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–33. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273:1933–7. [PubMed] [Google Scholar]
- 24.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 25.Mosman TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–88. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 26.Mosman TR, Coffman RL. Two types of mouse helper T-cell clone: implication for immune regulation. Immunol Today. 1987;8:223–9. doi: 10.1016/0167-5699(87)90171-X. [DOI] [PubMed] [Google Scholar]
- 27.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 28.Romagnani S. Biology of human Th1 and Th2 cells. J Clin Immunol. 1995;15:121–9. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- 29.Abas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 30.Kronin V, Hochrein H, Shortman K, Kelso A. Regulation of T cell cytokine production by dendritic cells. Immunol Cell Biol. 2000;78:214–23. doi: 10.1046/j.1440-1711.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 31.Heufler Ch, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman R, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper cells. Eur J Immunol. 1996;26:659–68. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 32.Sunder Plassmann G, Derfler K, Wagner L, et al. Increased serum activity of interleukin-2 in patients with pre-eclampsia. J Autoimmun. 1989;2:203–5. doi: 10.1016/0896-8411(89)90156-x. [DOI] [PubMed] [Google Scholar]
- 33.Hamai Y, Fujii T, Yamashita T, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-α levels before the clinical manifestations of pre-eclampsia. Am J Reprod Immunol. 1997;38:89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 34.Hara N, Fujii T, Okai T, et al. Histochemical demonstration of interleukin-2 in decidua cells of patients with pre-eclampsia. Am J Reprod Immunol. 1995;34:44–51. doi: 10.1111/j.1600-0897.1995.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 35.Dudley DJ, Hunter C, Mitchell MD, Varner M, Gately M. Elevations of serum interleukin-12 concentrations in women with severe pre-eclampsia and HELLP syndrome. J Reprod Immunol. 1996;31:97–107. doi: 10.1016/0165-0378(96)00976-x. [DOI] [PubMed] [Google Scholar]
- 36.Daniel Y, Kupferminc MJ, Baram A, Jaffa AJ, Fait G, Wolman I, Lessing JB. Plasma interleukin-12 is elevated in patients with pre-eclampsia. Am J Reprod Immunol. 1998;39:376–80. doi: 10.1111/j.1600-0897.1998.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 37.Sacks GP, Scott D, Tivnann H, Mire-Sluis T, Sargent IL, Redman CW. Interleukin-12 and pre-eclampsia. J Reprod Immunol. 1997;34:155–8. doi: 10.1016/s0165-0378(97)00028-4. [DOI] [PubMed] [Google Scholar]
- 38.Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subject and increased in pre-eclampsia. Am J Reprod Immunol. 2002;47:91–7. doi: 10.1034/j.1600-0897.2002.1o020.x. [DOI] [PubMed] [Google Scholar]
- 39.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with pre-eclampsia. Am J Reprod Immunol. 1998;40:102–11. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 40.Meekins JW. Interleukin-6, tumor necrosis factor and soluble tumor necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynecol. 1996;102:842–3. doi: 10.1111/j.1471-0528.1995.tb10859.x. [DOI] [PubMed] [Google Scholar]
- 41.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CWG. Interleukin-6, tumor necrosis factor and soluble tumor necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynecol. 1996;102:20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 42.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe pre-eclampsia. Am J Obstet Gynecol. 1994;170:1757–9. [PubMed] [Google Scholar]
- 43.Omu AE, Al Qattan F, Diejomaoh ME, Al Yatama M. Differential levels of T helper cytokines in preeclampsia: pregnancy, labor and puerperium. Acta Obstet Gynecol Scand. 1999;78:675–80. [PubMed] [Google Scholar]
- 44.Gratacós E, Filella X, Palacio M, Cararach V, Alonso PL, Fortuny A. Interleukin-4, interleukin-10 and granulocyte-macrophage colony stimulating factor in second-trimester serum from women with preeclampsia. Obstet Gynecol. 1998;92:849–53. doi: 10.1016/s0029-7844(98)00300-7. [DOI] [PubMed] [Google Scholar]
- 45.Santiago-Schwartz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferent activate TH1 inflamatory type responses. J Immunol. 2001;167:1758–68. doi: 10.4049/jimmunol.167.3.1758. [DOI] [PubMed] [Google Scholar]