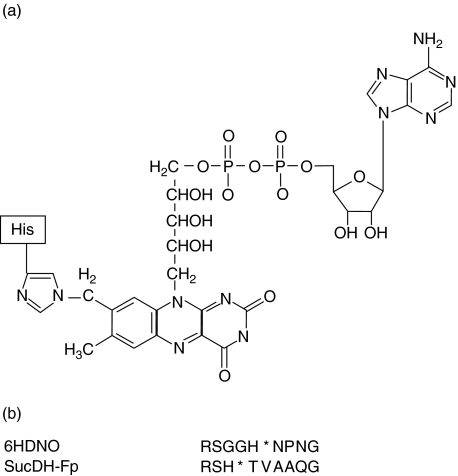
Fig. 1.
Structure of the naturally occurring FAD-hapten. (a) Covalent attachment of FAD via the 8α-group of the izoaloxazine ring of riboflavin to the N3 nitrogen of a histidine residue of the polypeptide chain. (b) Alignment of the amino acid residues surrounding the FAD-binding His (indicated by a star) of the bacterial flavoenzyme 6HDNO and of the mitochondrial SucDH Fp subunit.