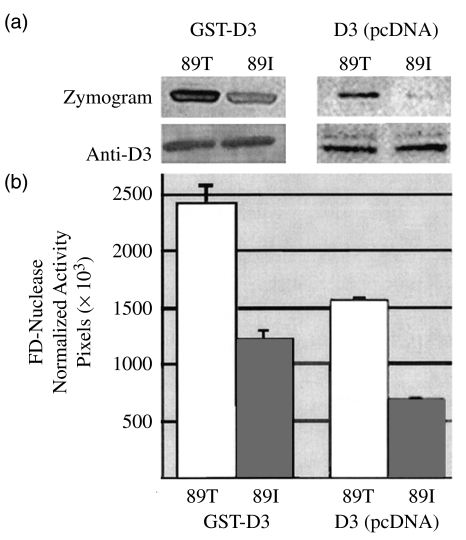
Fig. 2.
FD-nuclease activities of 89T and 89I Dnase1l3 s. (a) Corresponding zymograms and anti-Dnase1l3-peptide Western blots of Dnase1l3-GST fusions expressed in a prokaryotic system (E. coli, left) and Dnase1l3 expressed in eukaryotic cells (HeLa, right). Anti-Dnase1l3 immunoblots detect, respectively, the expected column-purified approximately 56 kD GST-fusions (left) or approximately 28 kD enzymes in lysates of cells transfected with pcDNA3·1-Dnase1l3. Zymograms (top) were performed on parallel and equal loads of the immunoblotted samples. While protein levels are nearly equal, the 89I enzyme has 53% the nuclease activity of the wild-type enzyme (n = 8, mean values 2419 ± 154 versus 1211 ± 84 pixels; Wilcoxon signed rank sum, P < 0·01). Zymogram activity was normalized relative to Dnase1l3-expression by western. (b) Graph of results.