Fig. 1.
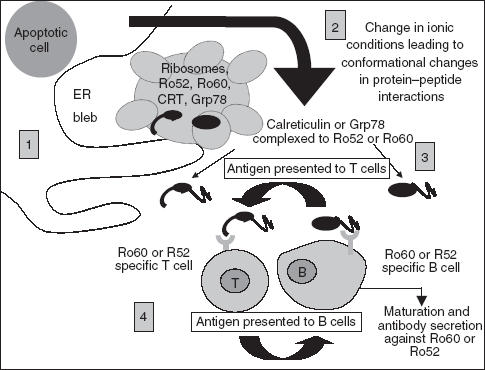
Model showing possible effects of stress protein interaction with Ro polypeptides. (1) Defective clearance of apoptotic blebs containing a combination of stress proteins and ribonuclear proteins could result in their release into the extracellular environment. (2) Changes in the ionic conditions may lead to conformational changes in the both the stress proteins and Ro autoantigens, leading to closer interaction with one another, resulting in structural modification and exposure of cryptic epitopes. (3) Such changes may impair the tolerance-inducing function of the apoptotic process leading to autoimmunity as the protein clusters are taken up by either Ro specific B or T cells. (4) After antigen processing, MHC class II molecules with peptides from the ER cluster of proteins are presented to either naïve Ro, Grp78 or calreticulin Th-cells or directly to B cell specific cells, ultimately leading to B cell maturation and secretion of autoantibodies against the protein clusters.
