Abstract
Autoantibodies against proteinase 3 (PR3) and myeloperoxidase (MPO) (ANCA = anti-neutrophil cytoplasmic antibodies) are used as diagnostic tools for patients with small vessel vasculitis. ANCA are detected by different assays, but the correlation between the results of these assays is generally poor. The overall aim of the study was to provide a framework for the future development of new assays with an increased diagnostic yield. In order to express discrete epitopes of human PR3 (hPR3), the nonantigenic molecules murine PR3 (mPR3) and human leucocyte elastase (HLE) were used as a framework. We constructed recombinant chimeric vectors and were able to produce 6 hPR3/mPR3 proteins and 3 hPR3/HLE proteins. Anti-PR3 monoclonal antibodies differed in their binding pattern to the chimeras, but no distinct binding region could be identified for any monoclonal antibody. The recombinant hPR3/mPR3 were also tested in ELISA with sera from patients with Wegener's granulomatosis with renal involvement. The results show that patients have antibodies to different constructs, indicating that the patients vary in their antibody repertoire from the beginning of the disease, and that patients may have antibodies from a broad range of clones early in the course of the disease. Recombinant hPR3/mPR3 chimeric proteins have a potential to be used as antigens in future ANCA assays.
Keywords: ANCA, proteinase 3, epitope, vasculitis, Wegener's granulomatosis
INTRODUCTION
Proteinase 3 (PR3) is a 29-kD neutral serine protease located in the azurophilic granulae of neutrophils and in granulae of monocytes. It was first described by Ohlsson and Olsson in 1973 [1]. PR3 undergoes several post-translational intracellular processing steps before it is stored in its enzymatically active form [2]. After removal of the propeptides, the mature PR3 enzyme consists of 221–222 amino acid residues [3]. PR3 has strong proteolytic activity and is able to degrade a number of extracellular matrix proteins including laminin, vitronectin, fibronectin and type IV collagen [4].
Autoantibodies against PR3 are called anti-neutrophil cytoplasmic antibodies (ANCA) since they were first recognized in indirect immunofluorescence (IIF) staining the cytoplasma of ethanol-fixed neutrophils [5]. This cytoplasmic staining pattern, now denoted as c-ANCA, was first reported in 1982 in eight patients with necrotizing glomerulonephritis [6], and in 1985 the association with active Wegener's granulomatosis (WG) was established [7]. Since then PR3-ANCA/c-ANCA has become an important diagnostic tool. Approximately 85% of patients with Wegener's granulomatosis and 45% of patients with microscopic polyangiitis (MPA) test positive for PR3-ANCA [8,9]. Relapses are frequently preceded by a rise in ANCA titre, and ANCA is used in the follow-up of patients with systemic vasculitis [10].
Various detection methods for PR3-ANCA are used, most frequently IIF and various enzyme-linked immunosorbent assays (ELISA). The results of these assays do not always correlate qualitatively no quantitatively. There are indications that these differences in methods have clinical implications. One example is a capture-PR3-ANCA ELISA, that has been developed at our laboratories [11]. This assay is shown to be more sensitive in the detection of patients with Wegener's granulomatosis as compared to standard ELISA with equal specificty [12], and more sensitive in the detection of relapses [13]. A plausible interpretation of this is that different epitopes on the PR3 antigen are exposed, depending on the assay, and that antibodies directed at certain epitopes have a higher diagnostic potential.
Several recent studies have been carried out to map the epitopes recognized by the PR3-ANCA. Synthetic peptides produced in an overlapping fashion spanning the whole PR3 molecule have been produced by several groups in order to find linear epitopes for PR3-ANCA [14–17]. Sera from patients with WG and MPA have been shown to react more strongly with some of these peptides in ELISA tests, but in three of the studies the same peptides were also recognized by control sera [15–17]. Furthermore, the results of these studies are inconclusive, since different regions have been pointed out as antigenic in the different studies.
An alternative approach is to express different parts of the antigenic molecule in a homologueous but nonantigenic framework. We have previously successfully used this approach in Goodpasture's disease to locate epitopes on the NC1 domain of the α3 chain of type IV collagen [18]. To this end we used chimeric molecules consisting of parts of the antigenic α3 chain and parts of the nonantigenic α1 chain. Recombinant PR3 recognized by patient sera has been successfully expressed in several cell lines [19–21]. In the present study we have utilized the similarities in sequence and shape between human PR3 (hPR3), human leucocyte elastase (HLE) and murine PR3 (mPR3) constructing recombinant chimeric molecules comprised of these proteins.
The aim of this study was to map the epitope specificities of anti-PR3 antibodies by using recombinant chimeric molecules of HLE and hPR3 and of hPR3 and mPR3.
MATERIALS AND METHODS
Patients and sera
Sera from three patients with the diagnosis Wegener's granulomatosis with biopsy-confirmed renal involvement were collected at the time of diagnosis and stored at −20°C until analysed. Sera from three healthy blood donors, stored at −20°C until analysed, were used as negative controls.
The study was approved by the Local Ethical Committee at the Faculty of Medicine, Lund University and informed consent was obtained from all the subjects in the study.
Antibodies and antigens
Three of the mouse monoclonal antibodies (mAbs) against PR3, 4A3, 4A5 and 6A6 were supplied by Wieslab AB, Lund, Sweden [22], and two, MCPR3-1 and MCPR3-2 [23], were previously developed by Dr U Specks. The control antibody, Mab17, is a mouse monoclonal antibody against the NC1 domain of the α3chain of type IV collagen [24]. PR3 from human neutrophils and the polyclonal rabbit anti-human PR3 antibodies were supplied by Wieslab AB.
The polyclonal rabbit anti-murine PR3 antibody was developed in Dr U Specks laboratory using purified recombinant murine PR3 expressed in HMC-1 cells as antigen [25]. The polyclonal rabbit anti-human elastase antibodies were purchased from Merck, Darmstadt, Germany. The monoclonal mouse antibody against the 6×His was purchased from Serotec, Kidlington, UK. The alkaline phosphatase-conjugated swine anti-rabbit IgG antibodies and rabbit anti-mouse IgG antibodies were purchased from DAKO, Glostrup, Denmark, and the alkaline phosphatase-conjugated goat anti-human IgG antibodies were purchased from Sigma (St Louis, MO, USA).
Expression vector
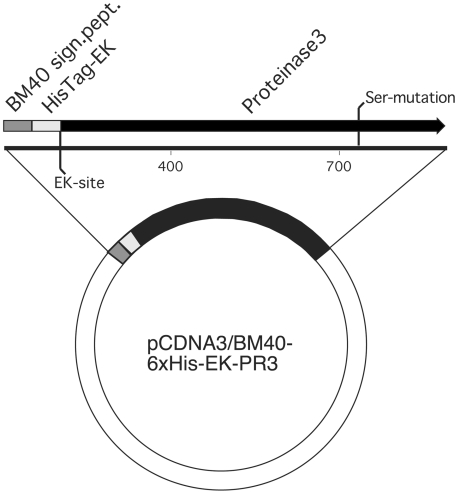
The expression vector used in this study, pcDNA3-BM40-HisEK, is shown in Fig. 1. The vector is based on the pcDNA3 expression vector (Invitrogen, Leek, the Netherlands) and adds a BM40 signal peptide, for exporting proteins to the medium, followed by a hexa-histidine tag, for purification, and an enterokinase D cleavage site N-terminal of the recombinant protein to cleave off the BM40 signal and Histidine-tag. It provides a cytomegalovirus promoter-driven expression of introduced cDNA and also carries a neomycine resistance gene, allowing for selection of transfected cells.
Fig. 1.
The expression vector pcDNA3-BM40-HisEK that is based on the mammalian CMV promoter-driven pcDNA3 vector. It adds a BM40 signal peptide for exporting proteins to the medium followed by a hexa-histidine tag for purification and an enterokinase D cleavage site N-terminal of the recombinant protein to cleave off the BM40 signal and histidine tag.
Construction of DNA for expression
Full length cDNA for human PR3, mutated at the active site by changing Ser203 to Gly to create an enzymatically inactive mutant, and full length cDNA for human elastase, was kindly provided by Dr U Gullberg at the Department of Haematology in Lund. Full length mouse PR3 was kindly provided by Dr L Hellman at the Department of Cell and Molecular Biology in Uppsala, Sweden. Using these cDNAs as templates in a PCR with primers constructed to exclude the signal peptides and propeptides, in order to make mature proteins, and to introduce a HindIII site at the 5′ end of the constructs and a NotI site at the 3′ end, new templates for the following constructs were made. Due to technical reasons, when inserting the Hind III site, the first isoleucine in all the recombinant proteins was exchanged for a leucine. All three amino acid sequences for these templates are shown in Fig. 2. Six constructs consisting of thirds of hPR3 and elastase and six constructs consisting of thirds of hPR3 and mPR3 were made using these molecules as templates and the 22 primers shown in Table 1 using overlap extension PCR technique described by Ho [26] (GeneAmp PCR System 2400, Perkin Elmer, Foster City, CA, USA). The Pfu-polymerase was purchased from Stratagene and the dNTPs and restriction enzymes from Roche.
Fig. 2.
(a, b) The amino acid sequences for human PR3 (hPR3), human leucocyte elastase (ELA) and murine PR3 (mPR3) used as templates for the recombinant proteins. The signal peptides and propeptides are removed and a HindIII site is introduced at the 5′ end and a NotI site is introduced at the 3′ end. All the recombinant molecules were mutated at the active site by changing Ser to Gly (shaded) to create enzymatically inactive mutants. The cleavage sites for the different constructs are marked a, b, c and d. (c) The chimeric constructs were named according to the origin of the respective proportions of the molecule, where P stands for hPR3, p for mPR3 and E for elastase. For example the PPp construct is comprised of hPR3 from a to c and the last part, from c to d, is mPR3 and the PEP construct is comprised of hPR3 from a to b, elastase from b to c and hPR3 from c to d. rhPR3 is recombinant hPR3, rmPR3 is recombinant mPR3 and rHLE is recombinant HLE.
Table 1.
Primers used in this study
| Primer | Sequence 5′ to 3′ |
|---|---|
| 1. | GCTAAAGCTTGTGGGCGGGCACGAGGCG |
| 2. | GTGCGGGTGGTCCTGGGA/GCCCACAACGTGCGGACG |
| 3. | AGCGTCCTGCAGGAGCTC/AATGTCACCGTGGTCACC |
| 4. | CCGCGAGAGGTTATGGGC/TCCGAGCACCACGTTCAC |
| 5. | CGTCACCACCGTCACGTT/GAGCCCCTGCAGGACCTG |
| 6. | GCTAGCGGCCGCTCAGGGGCGGCCCTTGGC |
| 7. | GCTAAAGCTTGTGGGGGGCCGGCGAGCG |
| 8. | GCCCATAACCTCTCGCGGCGG |
| 9. | AACGTGACGGTGGTGAC |
| 10. | TTCCAGGACCACCCGCACCGC |
| 11. | GAGCTCCTGCAGGACGCTGGC |
| 12. | GACTTCAGGCGGCCGCTCAGTGGGTCCTGCTGGCCGGGTCCG |
| 13. | CTTGTGACAGTGGTGCTG’GGAGCCCACAACGTGCGG |
| 14. | CAGGAACTGAACGTCACG’GTGGTCACCTTCTTCTGC |
| 15. | CAGCAGGTCGTGGGCACC’GAGCACCACGTTCACCAG |
| 16. | GCATAGGAAGGTGACCAC’GGTGACATTGAGCCCCTG |
| 17. | ACACAAAGCTTGTAGGTGGGCACG |
| 18. | CTGGTGAACGTGGTGCTC’GGTGCCCACGACCTGCTG |
| 19. | CAGGGGCTCAATGTCACC’GTGGTCACCTTCCTATGC |
| 20. | CCGCACGTTGTGGGCTCC’CAGCACCACTGTCACAAG |
| 21. | GCAGAAGAAGGTGACCAC’CGTGACGTTCAGTTCCTG |
| 22. | GTGTGTGCGGCCGCGCCCCAGCTTTAGGCTGC |
The protein coding cDNAs were then inserted in the pcDNA3-BM40-HisEK vector (Fig. 1). All constructs were sequenced using the AmpliTaq-FS sequencing kit (Perkin Elmer) and analysed with an ABI310 automated sequencer (Perkin Elmer).
Cell culturing and transfection
HEK-293 cells were cultured in 90-mm cell culture plates (TPP, Trasadingen, Switzerland) in a DMEM:F12 medium with 5% fetal calf serum (Gibco-BRL, Paisley, UK). For every construct 8 × 105 HEK-293 cells were transfected with 10 µg of plasmid using an electroporator (Bio-Rad, Hercules, CA, USA), with the electrical settings 200 V, 960 µF in a 0·4-cm cuvette.
After electroporation, the cells were seeded on new plates and after 48 h selection with geniticin was initiated. Transfected, geniticin resistant cells were cultured until a desired number of plates had reached confluence after which collection of supernatants was initiated. During harvesting, the transfected cells were kept in FCS-free DMEM:F12 supplemented with 50 mg/l ascorbate [18].
Purification of recombinant protein
Five hundred millilitres of harvested cell supernatant was centrifuged at 6200 × g for 20 min to remove cell debris. Proteins were precipitated overnight using (NH4)2SO4 at a concentration of 330 g/l medium with gentle stirring at 4°C and with addition of 1 mm PMSF. The precipitate was centrifuged at 6200 × g for 20 min The pellet was dissolved in 40 ml of starting buffer; 50 mm sodium phosphate pH 7·0, 0·3 m NaCl, 0·1% Triton X-100, and then applied to a FPLC column packed with 2·5 ml of Talon Superflow resin equilibrated in starting buffer. The column was washed with starting buffer until the baseline at 280 nm was stable. Bound material was eluted with 15 ml of 50 mm sodium phosphate pH 7·0, 0·5 m NaCl, 150 mm imidazol, 0·1% Triton X-100. Fractions were collected during the run and then analysed for PR3 and 6×His tag by ELISA.
The eluted samples were concentrated and dialysed against EKmax™ buffer (Invitrogen). The purified recombinant proteins were analysed by silver stained SDS-PAGE gels and by immunoblotting using antibodies against the His-tag. The expected size of recombinant proteins is approximately 29 kD. Protein concentrations were determined using the BCA (Pierce, Rockford, IL USA) method and by measuring the absorbance at 280 nm. The His-tag and BM40 signal peptide were then cleaved off by adding enterokinase 4 U/mg of recombinant protein overnight at 37°C. After this the recombinant proteins were re-analysed using silver stained SDS-PAGE and immunoblotting using antibodies against the His-tag to check that the His-tag was cleaved off.
Enzyme linked immunosorbent assay (ELISA)
Polystyrene microtitre plates (NUNC Immunoplate, Roskilde, Denmark) were coated overnight at room temperature with 0.1 µg/well of purified recombinant protein in coating buffer (50 mm Na2CO3, 0·05% NaH3, pH 9·6). The plates were washed three times with washing buffer (0·15 m NaCl, 0·05% (v/v) Tween 20) and then incubated for 1 h at room temperature (RT) with 100 µl/well of monoclonal antibodies or human sera diluted in PBS-BSA (1·5 mm KH2PO4, 8 mm Na2HPO4, 0·12 m NaCl, 2·5 mm KCl, 0·05% (w/v) NaN3 containing 0·02% (w/v) bovine serum albumin, pH 7·3). Human sera were diluted 1/100 and the mAb 4A3 1/100000, 4A5 1/125 000, 6A6 1/5000, MCPR3-1 1/100 000 and MCPR3-2 1/130 000. After three new washes the plates were incubated for 1 h with 100 µl alkaline phosphatase-conjugated rabbit anti-mouse IgG 1/1000 or goat anti-human IgG diluted 1/15 000 in PBS-BSA. The amount of bound antibodies was detected by the use of P-Nitrophenyl Phosphate (Sigma Chemical Company) (1 mg/ml) in substrate buffer (1 m Diethanolamine, 0·5 mm MgCl2, pH 9·8), as substrate. Colour development was measured spectrophotometrically at 405 nm after 1 h. All assays were done in duplicate.
Inhibition ELISA
Dilutions of mAbs adjusted to give the same absorption after 1 h in conventional ELISA with native PR3 were preincubated overnight at 4°C in a preincubation plate (NUNC lowbinding, Roskilde, Denmark) with the different inhibitors, i.e. recombinant proteins in concentrations varying from 0·08 to 10 µg/ml. The next morning they were transferred to polystyrene microtitre plates that had been coated with native PR3 in a concentration of 0·025 µg/well overnight and washed, and the ELISA was performed as above. Results were considered positive when 50% inhibition was achieved.
SDS-PAGE and immunoblotting
A 10% NuPAGE gel from Novex (San Diego, CA, USA) was run in a MOPS buffer system according to the supplier's recommendations. Gels were silverstained.
For immunoblotting, proteins were transferred to an Immibilon PVDF membrane (Millipore, St. Quentin, France), using a semidry electroblotter as described by Burnette [27]. The membranes were blocked with 2% BSA in coating buffer, and then washed with 0·15 m NaCl, 0·05% (v/v) Tween 20. The rabbit anti-hPR3 antibodies were diluted 1/500, the rabbit anti-mPR3 antibodies were diluted 1/1000 and the rabbit anti-elastase antibodies were diluted 1/200 and incubated for 2 h. After three washings the membranes were incubated for 1 h with secondary antibodies, swine anti-rabbit IgG-AP diluted 1/1000. The membranes were washed and then developed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indyl phosphate (Sigma).
RESULTS
Vectors with the genes coding for hPR3, mPR3 and HLE were constructed, as well as six chimeric vector constructs consisting of parts of hPR3 and HLE and six chimeric vector constructs consisting of parts of hPR3 and mPR3, as shown in Fig. 2. After transfection of the HEK-293 cells, all the chimeric hPR3/mPR3 constructs as well as the recombinant hPR3, mPR3 and HLE, yielded clones secreting recombinant protein, as measured by anti-HisTag ELISA, but despite of several attempts it was only possible to produce three of the hPR3/HLE constructs. To ensure that the recombinant proteins had the correct molecular weight, SDS-PAGE gels were run and silverstained (Fig. 3).
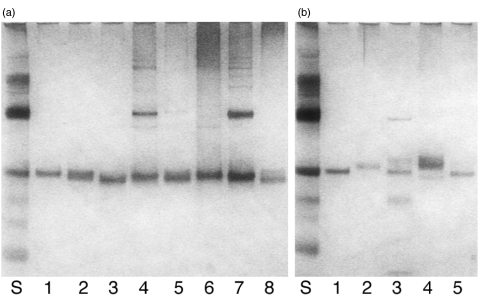
Fig. 3.
Silverstained gels showing the chimeric hPR3/mPR3 proteins in panel A and the hPR3/elastase proteins in panel B. In both panels lane 1 contains rhPR3. In panel A lanes 2–7 contain the chimeric proteins PPp, Ppp, PpP, pPp, pPP and ppP, with rmPR3 in lane 8, and in panel B lanes 2–4 contain the chimeric proteins PPE, PEE and PEP, with rHLE in lane 5.
Immunoblotting
When immunoblotting was performed on the hPR3/mPR3 proteins, all six chimeric proteins, as well as the recombinant hPR3 (rhPR3), were recognized by the polyclonal rabbit anti-hPR3 antibodies, but the recombinant mPR3 (rmPR3) was not (Fig. 4). All six chimeric proteins, as well as rmPR3, were recognized by the polyclonal rabbit anti-mPR3 antibodies, but rhPR3 was not recognized by these antibodies.
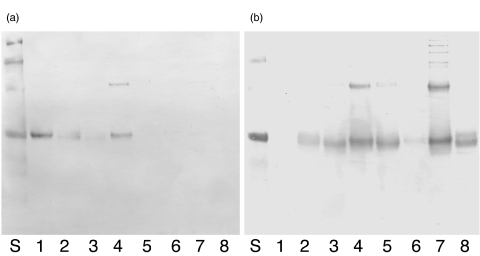
Fig. 4.
Western blots showing (a) binding of rabbit anti-human PR3 antibodies to the hPR3/mPR3 proteins and (b) the binding of rabbit anti-murine PR3 to the hPR3/mPR3 proteins. The order of the recombinant proteins is the same in both panels starting from lane 1 with rhPR3, and continuing through lanes 2–8 with PPp, Ppp, PpP, pPp, pPP, PpP and rmPR3.
When analysing the hPR3/HLE constructs by immunoblotting, all three constructs, as well as rhPR3, were recognized by the polyclonal rabbit anti-hPR3 antibodies, but the recombinant HLE (rHLE) was not (Fig. 5). All three constructs, as well as rHLE, were recognized by the polyclonal rabbit anti-elastase antibodies, while rhPR3 was not.
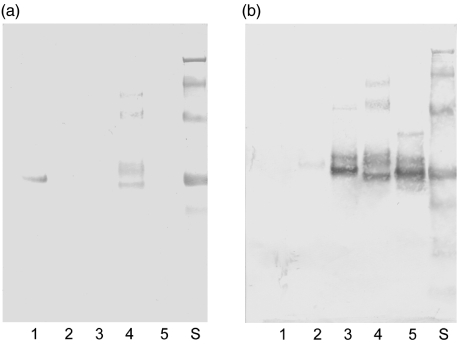
Fig. 5.
Western blots showing (a) binding of rabbit anti-human PR3 antibodies to the hPR3/elastase proteins and (b) the binding of rabbit anti-human elastase antibodies to the hPR3/elastase proteins. The order of the recombinant proteins is the same in both panels starting from lane 1 with rhPR3, and continuing through lanes 2–5 with PPE, PEE, PEP and rHLE.
Elisa
The five mAbs, 4A3, 4A5, 6A6, MCPR3-1 and MCPR3-2, were tested in direct ELISA for reactivity to each of the eight recombinant hPR3/mPR3 proteins and to the positive control human granulocyte PR3 (Table 2). As expected, none of the mAbs showed reactivity to rmPR3, but all five showed positive reactivity with rhPR3 and with the positive control. In addition all mAbs also recognized one or several other constructs. 4A3 and 4A5 show the same pattern recognizing the constructs rhPR3, PPp, PpP and pPP. 6A6 only recognizes rhPR3 and pPP. MCPR3-1 and MCPR3-2 react similarly, recognizing rhPR3, PPp and PpP. The control, mab17, did not show any reactivity either to the positive control or to the recombinant proteins. The results indicate that if more than one third of the hPR3 molecule is exchanged, the constructs can not be recognized by the mAbs. 6A6 is most sensitive in this aspect, but exchanging the first part of the molecule has least importance.
Table 2.
The reactivity in arbitrary values for the mabs 4A3, 4A5, 6A6, MCPR3-1 and MCPR3-2 to the eight hPR3/mPR3 recombinant proteins and native PR3 as measured by direct ELISA. Mab17 is a negative control
| 4A3 | 4A5 | 6A6 | MCPR3-1 | MCPR3-2 | mab17 | |
|---|---|---|---|---|---|---|
| rhPR3 | + + + | + + + | + + | + + + | + + + | – |
| PPp | + + | + | – | + | + + | – |
| PpP | + + + | + + + | – | + + | + + | – |
| Ppp | – | – | – | – | – | – |
| pPP | + + + | + + + | + | – | – | – |
| pPp | – | – | – | – | – | – |
| ppP | – | – | – | – | – | – |
| rmPR3 | – | – | – | – | – | – |
| PR3 | + + | + + | + + | + + | + + | – |
– below cut off; + 0·2–0·49 absorbance after 1 h at 405 nm; + + 0·5–0·99 absorbance after 1 h at 405 nm; + + + > 1·0 absorbance after 1 h at 405 nm.
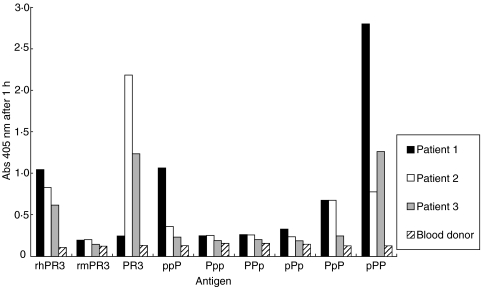
Sera from three patients with Wegener's granulomatosis with renal involvement, drawn at the time of diagnosis, and sera from three healthy blood donors were run in direct ELISA with the recombinant proteins. Results are shown in Fig. 6. Patient number 1 showed strong reactivity to the rhPR3, PpP, pPP and ppP constructs, patient number 2 showed strong reactivity to the rhPR3, PpP and pPP constructs while patient number 3 only showed reactivity to the rhPR3 and pPP constructs. Sera from the healthy blood donors (only one shown) did not show reactivity to any of the recombinant proteins.
Fig. 6.
ELISA results for sera from three patients with Wegener's granulomatosis (1–3) and serum from one healthy blood donor against hPR3/mPR3 constructs.
Inhibition ELISA with recombinant proteins and mabs
In the inhibition ELISA test, 50% inhibition of the constructs’ binding capacity was measured. All results are shown in Table 3. No mAb showed any reactivity with rmPR3. There were technical problems with the full length hPR3 in the inhibition ELISA assay. Inhibition seemed to be hampered by immune complex formation. The results are not in accordance with the results from the direct ELISA, for example 4A3 reacts strongly with PPp, Ppp and pPp in inhibition ELISA and with PpP and pPP in direct ELISA.
Table 3.
Inhibition ELISA against native purified PR3, using recombinant hPR3/mPR3 proteins and mabs 4A3, 4A5, 6A6, MCPR3-1 and MCPR3-2. Arbitrary values for concentrations giving 50% inhibition
| 4A3 | 4A5 | 6A6 | MCPR3-1 | MCPR3-2 | |
|---|---|---|---|---|---|
| rhPR3 | + | + | – | + | + |
| PPp | + + + | + + + | + | + + + | + + + |
| PpP | + + | + | – | + | + |
| Ppp | + + + | + + + | – | + | + |
| pPP | + + | + | + + | + | + |
| pPp | + + + | + + + | + | – | – |
| ppP | + | + | – | + | + |
| rmPR3 | – | – | – | – | – |
– no inhibition; + > 1·0 µg/ml; + + 0·08–0·99 µg/ml; + + + < 0·08 µg/ml.
DISCUSSION
The overall aim of our study was to provide a framework for the future development of new and better assays for the detection of PR3-ANCA. Our work is based on the assumption that antibodies directed against certain epitopes have a higher diagnostic potential. Moreover, the various assays expose different epitopes and thereby measure only a subpopulation of the polyclonal bulk of autoantibodies, thus explaining the different results given by the different methods. Earlier studies have indicated that the capture assay correlates better with disease activity, but new assays are necessary to increase the diagnostic yield.
Our hypothesis is supported by our previous studies concerning the clinical utility of our capture ELISA for PR3-ANCA [12,13]. In this assay the antigen is held in the microtiter plate by a capturing monoclonal antibody. The antigen is therefore presented in a more native conformation compared to the antigen coated directly into the plate. This more native way of displaying the antigen in the assay leads to a higher sensitivity with an equal specificity for the detection of renal vasculitis as compared to IIF and standard ELISA. Furthermore, it gives an increased sensitivity for the detection of relapses and a high titre in the capture ELISA correlated to patient survival [28]. No similar correlations were seen using standard ELISA. All these findings indicate that detection of autoantibodies directed to certain epitopes is more relevant than other autoantibodies.
There are now numerous in vitro studies and more recently animal experiments that support the notion that ANCA itself participates in the pathogenesis through the interaction between the autoantibodies and PR3 expressed on the surface of circulating neutrophils [29,30]. If this notion is correct it is reasonable to believe that only epitopes available on surface-PR3 are interesting to measure, putting further emphasis on the importance of more knowledge about epitope specificity of the PR3-ANCA and their pathological relevance and relation to disease activity.
Our hypothesis regarding the relevance of different epitopes stems from our work with antibodies against glomerular basement membrane (GBM) [18]. In glomerulonephritis, many reactivities can be measured against GBM in vitro, but only antibodies to the NC1 domain of type IV collagen are diagnostic for Goodpasture's disease. Furthermore, among NC1 antibodies only antibodies directed to the α3 chain have proven to be important, and among those only antibodies directed to a certain epitope region in the N-terminal third of the domain [31].
In our present study we adopted an approach similar to our work with anti-GBM. In order to express discrete epitopes we used a nonantigenic molecule with a structure similar to PR3 as a framework. By substituting parts of PR3 for parts of the nonantigenic molecule we hope to construct a molecule, displaying active vasculitis relevant epitopes only. The expression of recombinant antigens was done in human embryonic kidney cells (HEK-293) that are known to provide a complete machinery for post-transcriptional modifications and that also secrete large amounts of protein to the medium. We started out using HLE, which has a 53% sequence homologuey with hPR3, as the framework molecule. Six different chimeric constructs were made, but we were only able to produce three of these hPR3/HLE proteins in sufficient amounts. We do not believe that this was for technical reasons since we made different vectors, and tried several transfection and culture conditions. The most probable explanation is that these chimeric molecules were malfolded with consequent degradation in the ER. Instead, we decided to use mPR3, which has a 65% sequence homologuey with hPR3. This approach was more successful and all six chimeric hPR3/mPR3 proteins were produced, exported to the culture medium and appeared to have the correct molecular weight by Western blot.
After purification the recombinant proteins were tested in ELISA. The anti-PR3 monoclonal antibodies differed in their binding pattern to hPR3/mPR3, but no distinct region for their binding could be identified. For example 4A3 showed reactivity to PPp as well as to PpP and pPP in the direct ELISA. We interpret this as meaning that the amino acids making up the binding site for the monoclonal antibodies are present in the human as well as the murine PR3 sequence, but mPR3 is lacking the correct tertiary structure to bring these amino acids together. To avoid these problems, in the further characterization of the epitopes, one possibility is to express the negative backbone, i.e. mPR3 or HLE, with only small areas exchanged to hPR3. The selected amino acids should be situated close together on the surface when the molecule is correctly folded.
The results in the inhibition ELISA, where the antigen is free in solution, differed from the standard ELISA, which provides further evidence for the importance of the assay used to detect PR3-ANCA. The mAbs did not recognize the hPR3/HLE constructs, supposedly because of a very disturbed tertiary structure. The only chimeric hPR3/HLE molecule recognized by the mAbs and giving reproducible results had one third elastase in the C-terminal part (EPP, data not shown).
Apart from the mAbs we also tested the recombinant hPR3/mPR3 with sera from patients with Wegener's granulomatosis with renal involvement drawn at the time of diagnosis. This was done in a direct ELISA and the results show that the patients have antibodies to different constructs, suggesting that patients vary in their PR3-ANCA repertoire from the beginning of the disease, and that patients may have antibodies from different clones early in the course of the disease. This finding is based on very few samples but may, if it could be confirmed in a larger cohort, be of diagnostic importance. We intend to pursue this systematically in future studies by analysing samples from a larger group of patients drawn at different stages of disease.
In this study we have shown that chimeric molecules constructed from hPR3 and mPR3 can be expressed as recombinant proteins, and that they are recognized both by PR3-ANCA from patients and by monoclonal antibodies against PR3. The approach of exchanging thirds of hPR3 for mPR3 has not revealed the exact localization of the epitopes of the mAbs, but as patient PR3-ANCA recognizes them differently, assays based on these chimeric molecules may still harbour a diagnostic potential.
Acknowledgments
The authors thank Dr U. Specks for generously providing the monoclonal antibodies (MCPR3-1 and MCPR3-2) and the polyclonal rabbit anti-mouse-PR3 antibody. This study was supported by grants from the Swedish Medical Research Council (project K-2002–74X-09487–12), the Crafoord foundation, the Tegger foundation, the Swedish Society for Medical Research and Riksförbundet för Njursjuka.
REFERENCES
- 1.Ohlsson K, Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of two granulocyte collagenases. Eur J Biochem. 1973;36:473–81. doi: 10.1111/j.1432-1033.1973.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 2.Garwicz D, Lindmark A, Hellmark T, Gladh M, Jogi J, Gullberg U. Characterization of the processing and granular targeting of human proteinase 3 after transfection to the rat RBL or the murine 32D leukemic cell lines. J Leukoc Biol. 1997;61:113–23. doi: 10.1002/jlb.61.1.113. [DOI] [PubMed] [Google Scholar]
- 3.Fujinaga M, Chernaia MM, Halenbeck R, Koths K, James MNG. The crystal structure of PR3, a neutrophil serine proteinase antigen of Wegener's granulomatosis antibodies. J Mol Biol. 1996;261:267–78. doi: 10.1006/jmbi.1996.0458. [DOI] [PubMed] [Google Scholar]
- 4.Rao NV, Wehner NG, Marshall BC, Gray WR, Gray BH, Hoidal JR. Characterization of proteinase-3 (PR-3), a neutrophil serine proteinase. Structural and functional properties. J Biol Chem. 1991;266:9540–8. [PubMed] [Google Scholar]
- 5.Savige J, Gillis D, Benson E, et al. International Consensus Statement on Testing and Reporting of Antineutrophil Cytoplasmic Antibodies (ANCA) Am J Clin Pathol. 1999;111:507–13. doi: 10.1093/ajcp/111.4.507. [DOI] [PubMed] [Google Scholar]
- 6.Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J Clin Res Ed. 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, van der Giessen M, van der Hem GK. The TH: autoantibodies against neutrophils and monocytes: Tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen Tervaert JW, van der Woude FJ, Fauci AS, et al. Association between active Wegener's granulomatosis and anticytoplasmic antibodies. Arch Intern Med. 1989;149:2461–5. doi: 10.1001/archinte.149.11.2461. [DOI] [PubMed] [Google Scholar]
- 9.Kallenberg CG, Brouwer E, Weening JJ, Tervaert JW. Anti-neutrophil cytoplasmic antibodies. current diagnostic and pathophysiological potential. Kidney Int. 1994;46:1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- 10.Cohen Tervaert JW, Goldschmeding R, Elema JD, et al. Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int. 1990;37:799–806. doi: 10.1038/ki.1990.48. [DOI] [PubMed] [Google Scholar]
- 11.Baslund B, Segelmark M, Wiik A, Szpirt W, Petersen J, Wieslander J. Screening for anti-neutrophil cytoplasmic antibodies (ANCA) is indirect immunofluorescence the method of choice? Clin Exp Immunol. 1995;99:486–92. doi: 10.1111/j.1365-2249.1995.tb05577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westman KW, Selga D, Bygren P, Segelmark M, Baslund B, Wiik A, Wieslander J. Clinical evaluation of a capture ELISA for detection of proteinase-3 antineutrophil cytoplasmic antibody. Kidney Int. 1998;53:1230–6. doi: 10.1046/j.1523-1755.1998.00873.x. [DOI] [PubMed] [Google Scholar]
- 13.Gisslen K, Wieslander J, Westberg G, Herlitz H. Relationship between anti-neutrophil cytoplasmic antibody determined with conventional binding and the capture assay, and long-term clinical course in vasculitis. J Intern Med. 2002;251:129–35. doi: 10.1046/j.1365-2796.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- 14.Griffith ME, Coulthart A, Pemberton S, George AJT, Pusey CD. Anti-neutrophil cytoplasmic antibodies (ANCA) from patients with systemic vasculitis recognize restricted epitopes of proteinase 3 involving the catalytic site. Clin Exp Immunol. 2001;123:170–7. doi: 10.1046/j.1365-2249.2001.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams RC, Jr, Staud R, Malone CC, Payabyab J, Byres L, Underwood D. Epitopes on proteinase-3 recognized by antibodies from patients with Wegener's granulomatosis. J Immunol. 1994;152:4722–37. [PubMed] [Google Scholar]
- 16.Van Der Geld YM, Simpelaar A, Van Der Zee R, Cohen Tervaert JW, Stegeman CA, Limburg PC, Kallenberg CGM. Antineutrophil cytoplasmic antibodies to proteinase 3 in Wegener's granulomatosis: epitope analysis using synthetic peptides. Kidney Int. 2001;59:147–59. doi: 10.1046/j.1523-1755.2001.00475.x. [DOI] [PubMed] [Google Scholar]
- 17.Chang L, Binos S, Savige J. Epitope mapping of anti-proteinase 3 and anti-myeloperoxidase antibodies. Clin Exp Immunol. 1995;102:112–9. doi: 10.1111/j.1365-2249.1995.tb06644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellmark T, Segelmark M, Unger C, Burkhardt H, Saus J, Wieslander J. Identification of a clinically relevant immunodominant region of collagen IV in Goodpasture disease. Kidney Int. 1999;55:936–44. doi: 10.1046/j.1523-1755.1999.055003936.x. [DOI] [PubMed] [Google Scholar]
- 19.Specks U, Fass DN, Fautsch MP, Hummel AM, Viss MA. Recombinant human proteinase 3, the Wegener's autoantigen, expressed in HMC-1 cells is enzymatically active and recognized by c-ANCA. FEBS Lett. 1996;390:265–70. doi: 10.1016/0014-5793(96)00669-2. [DOI] [PubMed] [Google Scholar]
- 20.Harmsen MC, Heeringa P, van der Geld YM, Huitema MG, Klimp A, Tiran A, Kallenberg CGM. Recombinant proteinase 3 (Wegener's antigen) expressed in Pichia pastoris is functionally active and is recognized by patient sera. Clin Exp Immunol. 1997;110:257–64. doi: 10.1111/j.1365-2249.1997.tb08325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szymkowiak CH, Johnston TW, Csernok E, Gross WL. Expression of the human autoantigen of Wegener's granulomatosis (PR3) in a baculovirus expression system. Biochem Biophys Res Commun. 1996;219:283–9. doi: 10.1006/bbrc.1996.0224. [DOI] [PubMed] [Google Scholar]
- 22.Sommarin Y, Rasmussen N, Wieslander J. Characterization of monoclonal antibodies to proteinase-3 and application in the study of epitopes for classical anti-neutrophil cytoplasm antibodies. Exp Nephrol. 1995;3:249–56. [PubMed] [Google Scholar]
- 23.Sun J, Fass DN, Hudson JA, Viss MA, Wieslander J, Homburger HA, Specks U. Capture-ELISA based on recombinant PR3 is sensitive for PR3-ANCA testing and allows detection of PR3 and PR3-ANCA/PR3 immunecomplexes. J Immunol Meth. 1998;211:111–23. doi: 10.1016/s0022-1759(97)00203-2. [DOI] [PubMed] [Google Scholar]
- 24.Johansson C, Butkowski R, Wieslander J. Characterization of monoclonal antibodies to the globular domain of collagen IV. Connect Tissue Res. 1991;25:229–41. doi: 10.3109/03008209109029159. [DOI] [PubMed] [Google Scholar]
- 25.Jenne DE, Frohlich L, Hummel AM, Specks U. Cloning and functional expression of the murine homologue of proteinase 3: implications for the design of murine models of vasculitis. FEBS Lett. 1997;408:187–90. doi: 10.1016/s0014-5793(97)00418-3. [DOI] [PubMed] [Google Scholar]
- 26.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–9. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 27.Burnette WN. ‘Western blotting’. electrophoretic transfer of proteins from sodium dodecyl sulfate – polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 28.Westman KW, Bygren PG, Olsson H, Ranstam J, Wieslander J. Relapse rate, renal survival, and cancer morbidity in patients with Wegener's granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol. 1998;9:842–52. doi: 10.1681/ASN.V95842. [DOI] [PubMed] [Google Scholar]
- 29.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–9. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–63. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellmark T, Burkhardt H, Wieslander J. Goodpasture disease. Characterization of a single conformational epitope as the target of pathogenic autoantibodies. J Biol Chem. 1999;274:25862–8. doi: 10.1074/jbc.274.36.25862. [DOI] [PubMed] [Google Scholar]