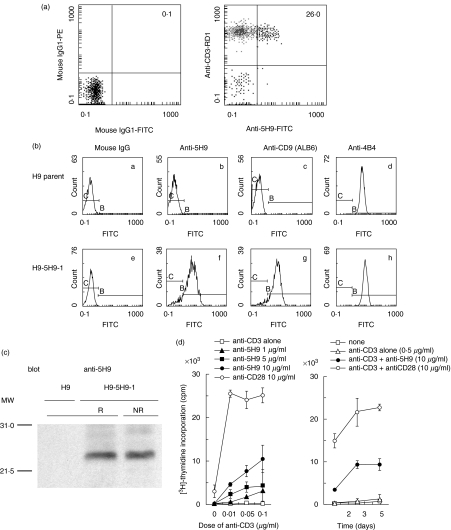
Fig. 1.
5H9 antigen is identified to be the CD9 molecule. (a) Reactivity of anti5H9 MoAb to human peripheral blood T cells. Two-colour staining analysis of freshly isolated human PBMC was performed using FITC-conjugated anti5H9, RD1-conjugated anti-CD3. Numbers indicate the relative percentages of positive cells within a quadrant. The result is representative of five separate experiments. (b) Reactivity of anti-CD9 MoAbs on H9 cells transfected with isolated cDNA of the 5H9 antigen. H9 parent cells as a negative control (a–d) or H9–5H9-1 cells transfected with isolated cDNA of 5H9 antigen (e–h) were stained with mouse IgG (a and e), anti5H9 (b and f), ALB6 (anti-CD9) (c and g), 4B4 (anti-CD29) (d and h) and FITC-conjugated goat antimouse IgG. (c) Western blotting of 5H9 antigen. H9–5H9-1 cells were lysed in lysis buffer. The lysates were separated on 12% SDS-PAGE under reducing (R) or non-reducing (NR) condition, and then immunoblotting was carried out with anti5H9 (blot). The positions of molecular weight markers are indicated on the left (MW). (d) Co-stimulatory effect of anti5H9 with the immobilized submitogenic dose of anti-CD3 on human peripheral blood T cells. Left: dose–response curve; right: time–course curve. Purified T cells at 2·0 × 105 cells/well were stimulated with immobilized suboptimal concentration of anti-CD3 (OKT3) and the indicated concentrations of anti5H9 or anti-CD28 (4B10). Proliferation was assessed by [3H]-thymidine (1 µCi/well) incorporation assay in the last 13 h of cultures. The result is representative of three independent experiments and expressed as mean cpm ± s.d. of triplicate cultures.