Abstract
The aim of this study was to assess the synovial fluid (SF) neurotransmitter excitatory amino acid (EAA) levels, including glutamate (Glu) and aspartate (Asp), in the context of SF levels of other amino acids, TNF-α and chemokines from patients with active arthropathies. The SF was collected from patients with active rheumatoid arthritis (RA), gout, or osteoarthritis (OA). The SF samples were analysed for levels of neurotransmitters glutamate and aspartate, tumour necrosis factor-alpha (TNF-α), Regulated upon Activation Normally T-cell Expressed and Secreted (RANTES), macrophage inhibitory factor-1 alpha (MIP-1α) and interleukin 8 (IL-8). SF WBC counts were also determined. Correlations between SF EAA, TNF-α and chemokines were determined by the Pearson product-moment correlation. Primary cultures derived from SF from active RA and gout patients were incubated with added l-glutamate, to assess if exposure to Glu could increase TNF-α levels. There were significant elevations in SF EAA, SF TNF-α and SF RANTES in RA patients compared to gout or OA patients. Significant correlations between SF EAA and SF RANTES, MIP-1α and IL-8 levels were seen, and SF EAA and SF TNF-α or SF WBC levels approached significance. Addition of exogenous neurotransmitter glutamate significantly increased TNF-α levels in primary cell cultures derived from RA and gout patients. The SF neurotransmitter EAA levels significantly correlated to selected SF chemokine levels, in clinically active RA, gout and OA patients, independent of disease. Added Glu resulted in significantly increased TNF-α levels in primary synovial cell cultures. These data expand the relationship of SF neurotransmitter EAA levels to SF cytokines and chemokines in patients with clinically active arthritis, and suggest that neurotransmitters Glu and Asp contribute to peripheral inflammatory processes.
Keywords: arthritis, glutamate, aspartate, TNF-α, chemokines
INTRODUCTION
Previous studies have demonstrated increased neurotransmitter excitatory amino acids (EAA), glutamate (Glu) and aspartate (Asp), in patients with active arthropathies [1]. Synovial fluid (SF) EAA levels were independent of SF WBC count or diagnosis when single time-point samples were analysed. Studies analysing amino acid levels in the context of body compartments have demonstrated that the SF EAA levels could be independent of plasma or other body fluid values. Independent SF EAA levels demonstrating compartmentalization are possibly due to local production, compartment accumulation or altered clearing mechanisms [2]. Additional studies analysing neurotransmitter EAA from sequential arthrocenteses demonstrated dynamic fluctuations of SF EAA over time, again reflecting local inflammatory processes [3,4]. The elevations in SF EAA, especially Glu, in experimental arthritis models have been associated with increased oedema and sensitization to thermal hyperalgesia [5,6].
Previous studies and reviews have reported increased SF cytokine levels notably TNF-α and IL-1 in patients with rheumatoid arthritis [RA] [7,8]. Production of SF TNF-α and inflammatory chemokines are thought to be from resident synovial cells and the cells migrating from the blood into the joint during inflammation [7–10]. Elevations in these cytokines are believed to stimulate the production of interleukin-8 (IL-8) and other chemokines, resulting in an influx of inflammatory cells into the joint space. Increased chemokine levels of Regulated upon Activation Normally T-cell Expressed and Secreted (RANTES) (macrophage inflammatory protein-1α (MIP-1α) and IL-8 have been reported in SF from active arthropathies [11–14].
This study compares the mean SF neurotransmitter EAA, TNF-α and chemokine (RANTES, MIP-1α and IL-8) levels in patients with RA, acute gout (gout) and osteoarthritis (OA), to evaluate possible correlations between the levels of SF EAA and the traditional inflammatory mediators reported in active arthritis. In addition, primary cell cultures derived from the SF of RA and gout patients were established to determine the effect of added l-glutamate on the production of TNF-α. These data support the hypothesis that increased SF neurotransmitter Glu and Asp levels contribute to joint inflammation in active arthropathies, such as RA and gout and OA.
MATERIALS AND METHODS
Patients
All patients had active arthritis during clinical evaluations, as determined by synovial effusion and pain. All patients fulfilled the 1987 ACR revised criteria for their diagnosis [15], including RA (n = 19). Synovial fluid (SF) from patients with acute gout (n = 15) contained phagocytosed monosodium urate crystals observed by polarized light microscopy and had negative cultures for microbial organisms. The OA was diagnosed clinically and all patients (n = 10) had correlative radiological changes of the affected joint with negative SF examinations for crystals and microbial organisms. Retrospective chart reviews (TM and BAB) of these patients were carried out independently to confirm the clinical diagnoses. The mean age ± SE and (age range) for the patient groups are as follows: RA: 53·5 ± 2·36 (30–70) years; gout: 54 ± 2·6 (43–65) years and OA: 58·3 ± 3·53 (47–68) years. The percentage of female patients for each group are as follows: RA: 63%, gout: 14% and OA: 40%.
Synovial fluids
Synovial fluids (SF) were collected from patients who underwent diagnostic or therapeutic arthrocenteses from 1993 to 2003. These discarded samples are maintained as part of a serology/fluids repository by the Gulf Coast Arthritis Registry-Serology (GCARS) at the University of Texas Medical Branch, Galveston, TX and are under IRB guidelines and approval. Synovial fluids were collected in sterile tubes after cell pelleting with no preservatives and stored at −20 or −80 degrees under sterile conditions. One-ml aliquots were also collected and transferred to microcentrifuge tubes to be analysed in the various assays under blinded conditions. Synovial fluids were thawed only once for all analyses.
High pressure liquid chromatography (HPLC)
Free amino acid determinations were obtained with a Waters 717 system with autosampler (Waters, Milford, MA USA), Beckman 114M solvent delivery pumps (Beckman Fullerton, CA USA), Bioanalytical systems FL-45 fluorescence detector (excitation 250 nm, emission 456: Bioanalytical systems, West Lafayette, LA USA) and Waters Millennium software. A standard HPLC protocol was utilized [1]. Samples (100 µl) were injected into the HPLC analyser with the reagent solution (sodium borate, 0·01 m, pH 8·95, Millipore filtered) and run at 25°C.
Experimental standards and quality control
Internal amino acid controls included homocysteine and norleucine. The recommended internal control is norleucine, as homocysteine competes with another protein peak in some rheumatoid arthritis samples. Internal sample controls were run after every 25 samples as quality controls to monitor inter- and intra-assay variability. Duplicate or triplicate samples were run for each patient. Control samples were included to confirm the negligible effects of heparin, EDTA, citrate, addition of whole blood and freeze-thawing up to 5 times on SF amino acid concentrations (data not shown). The SF samples were thawed once at the time of the assay. Previous studies showed no impact of deproteination (data not shown) or SF WBC count (1) on SF EAA levels.
Measurement of Synovial fluid TNF-α, RANTES, MIP-1α and IL-8
Quantitative measurements of SF TNF, RANTES, MIP-1α, and IL-8 were performed using a commercial immunoassay kit (Quantikine, R & D Systems, Minneapolis, MN, USA) expressed as pg/ml. SF samples were thawed × 1. Values were expressed as the average plus standard error for the groups or cell culture conditions.
The sensitivities and (ranges) for each immunoassay kit are provided from the manufacturer as follows: TNF-α: 4·4 pg/ml (15·6–1000 pg/ml), RANTES: 8 pg/ml (31·2–2000 pg/ml), MIP-1α: 10 pg/ml (46·9–1500 pg/ml for serum), IL-8: 10 pg/ml (31·2–2000 pg/ml).
For reference, the mean cadaver SF TNF-α concentration = 1·25 pg/ml, range 0–5 pg/ml and the mean SF RANTES levels from cadavers were negligible, i.e. below the levels of detection. The SF cadaver values for MIP-1α and IL-8 were not determined.
White blood cell counts
WBC counts derived from patient SF at the time of arthocentesis were determined by the hospital laboratory and are used as diagnostic or therapeutic information in the patient medical record. WBC counts are expressed as the cell number/mm3.
Cell cultures
Freshly obtained synovial fluid (30–40 mls of discarded specimen) from clinically active RA or gout patients were plated into tissue culture well plates (Corning, Corning, NY, USA) within 30 min of diagnostic or therapeutic knee arthrocentesis [16]. The culture plates were incubated overnight at 37°C, in 5% CO2. The next morning (16 h), the nonadherent cells (lymphocytes and polymorphonuclear leucocytes) were removed to isolate adherent cells (monocytes and synoviocytes) in culture. Adherent cells were washed with RPMI media (Gibco, Grand Island, NY, USA) ×3 and incubated with RPMI culture media, 2 mm L-Glutamine and 1000 units penicillin and 100 units streptomycin (Gibco). In addition, the cultures were incubated with 10% cell free synovial fluid from the same donor for the first 24 h. Parallel adherent cell culture wells were used for CD14 staining of the adherent cells, to determine the percentage of CD14+ (monocyte-derived) cells in the population. The adherent cells were 10–14% CD14 + cells by cell staining from both RA and acute gout SF. The adherent cell cultures were washed × 3 with PBS and replacement media now had 15% heat-inactivated fetal calf serum. On Day 7, aliquots of the culture media were taken. Experimental conditions were set up in triplicate. The exchange media included no added l-glutamate (control) or supplemented 500 µm (for RA) or 250 µm (for gout) l-glutamate (Sigma Chemical Co, St. Louis, MO, USA). The l-glutamate solution was pH adjusted to 7·4 with NaOH before addition to cultures). At two hours, culture supernatants were collected for EAA and TNF-α concentration determinations. The cultures were then washed with PBS × 2 and the media replaced with the culture media without added l-glutamate. Cell viability was confirmed at several time points on parallel cultures with trypan blue exclusion. At 15 h, a second set of culture supernatant aliquots was collected and the cells of the cultures checked for cell viability by trypan blue exclusion. The adherent cell cultures maintained >95% viability by trypan blue exclusion at both time points.
Data expression and statistical analyses
Mean SF EAA, TNF-α, RANTES, MIP-1α and IL-8-values are expressed as the mean concentrations with standard error (SE) from patient groups based on the diagnoses of RA, gout, or OA. Analyses of mediators were based on available SF patient sample numbers as follows: SF Glu and Asp: n = 44; SF TNF-α: n = 41; SF RANTES: n = 22; SF MIP-1α: n = 36; SF IL-8: n = 35 and SF WBC: n = 35. Correlation analyses among all SF AA, TNF-α, chemokine concentrations and WBC counts were evaluated by the Pearson product-moment correlation using the Statistica Version 5·0 program (Stat Soft Inc, Tulsa, OK). Unpaired and paired students t-tests were also performed using Sigmaplot software (SPSS, Inc, Chicago, IL, USA). A P-value <0·05 was considered significant.
RESULTS
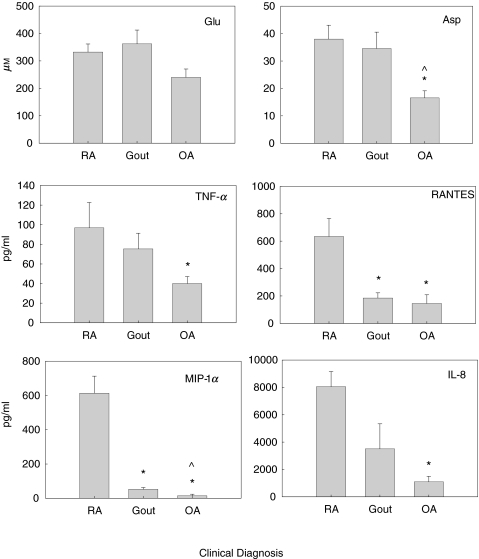
Figure 1 depicts the mean SF EAA concentrations for Glu and Asp (µm), TNF-α, RANTES, MIP-1α and IL-8 (in pg/ml) for clinically active RA, gout and OA patient samples. The mean SF Glu levels were 33% higher in RA and gout patient samples (inflammatory arthropathies) compared to OA patients (332·3 ± 29·30, 364·21 ± 50·19 and 240 ± 38 µm, respectively) but were not significant. The mean SF Asp levels were twofold higher in RA and gout patients, compared to OA patients (38·56 ± 5·05, 34·66 ± 5·96 and 16·87 ± 3·29 µm, respectively). The differences in mean SF Asp levels were significant between RA and OA groups (P < 0·01) and gout and OA groups (P < 0·02). The differences in SF Asp levels between RA and gout patient samples were not significant. The mean SF TNF-α concentrations were almost twofold higher in RA and gout patient samples, compared to OA patient samples (93·53 ± 25·21, 75·36 ± 15·92 and 38·88 ± 5·98 pg/ml, respectively). The differences in mean SF TNF-α levels were significant between RA and OA patient groups (P = 0·01). The difference in mean SF TNF-α levels between RA and gout samples did not reach statistical significance, nor did the differences in SF TNF-α levels between gout and OA patient samples.
Fig. 1.
Mean SF levels were determined for SF EAA Glu and Asp by HPLC, and SF TNF-α, RANTES, MIP-1α and IL-8 levels were determined by immunoassay (ELISA). SF levels were obtained for the following patients during clinically active disease; rheumatoid arthritis (RA, n = 19), gout (n = 15) and osteoarthritis (OA, n = 10). *P < 0·05 when compared to RA. ^P < 0·05 when compared to gout.
The differences in mean SF RANTES concentrations were also highest in RA samples compared to gout and OA (634·5 ± 132·96, 185 + 38·82 and 145 + 63·56 pg/ml, respectively). The mean SF RANTES levels in RA patients were significantly elevated compared to OA patient (P = 0·01) and gout patient samples (P = 0·03). The differences in mean RANTES levels between gout and OA patient samples were not significantly different. The SF sample number from gout patients (n = 3) was very low and may not reflect a meaningful comparison.
Markedly elevated mean SF MIP-1α concentrations were seen in RA, relative to concentrations seen in gout and OA patient samples (612·85 ± 109·86, 47·73 ± 10·76 and 14 ± 5·42 pg/ml, respectively). The differences in mean SF MIP-1α levels were significant between patient samples for RA versus OA (P < 0·01), RA versus gout (P < 0·001) and gout versus OA (P = 0·023). The mean MIP-1α level for OA was below the recommended range for the immunoassay, or negligible. Mean SF IL-8 levels were also highest in RA samples compared to gout and OA (8050 ± 1108, 3510·75 ± 1823 and 1096·1 ± 484 pg/ml, respectively). The mean SF IL-8 levels in RA patients were significantly elevated compared to OA (P < 0·001). The differences of mean SF IL-8 levels between RA and gout and gout and OA patients were not significant.
RA patients had the highest mean concentrations for all mediators studied, except for SF Glu, which was marginally higher in gout patients (332·32 ± 29·44 versus 364·21 ± 50·19 µm, respectively). There were variable mean SF chemokine levels relative to high SF EAA levels in the gout patient samples. The mean SF EAA and TNF-α levels were higher relative to those mean values obtained for SF RANTES, SF MIP-1α and SF IL-8 in the gout patient samples. In the OA patient samples, the SF EAA and SF TNF-α levels were comparatively higher than those of SF RANTES, SF MIP-1α and SF IL-8.
The mean ± standard error SF WBC counts for RA, gout and OA patient samples were 14 855·81 ± 1790·62, 15 708·3 ± 5129 and 1199·12 ± 420/mm3, respectively. These data are consistent for cell counts derived from ‘inflammatory arthropathies’, such as RA and acute gout and ‘noninflammatory’ arthropathies, such as osteoarthritis.
The mean SF EAA, TNF-α, RANTES levels and WBC counts were significantly elevated (P < 0·0001) in all SF samples derived from active arthropathies, compared to the mean values obtained from nonarthritic, cadaveric SF samples (not shown). Cadaveric SF values for MIP-1α and IL-8 were not obtained.
Correlation of SF EAA levels to levels of inflammatory mediators
Table 1 summarizes the correlation analyses demonstrating statistically significant associations between the SF EAA, inflammatory mediators and WBC counts, independent of disease. SF Glu levels were significantly correlated with SF Asp levels (P < 0·01), SF RANTES levels (P = 0·03) and SF IL-8 levels (P < 0·01). Mean SF Asp levels were significantly correlated with SF Glu (P < 0·01), SF RANTES (P < 0·01) and SF MIP-1α (P < 0·01). In addition, there were significant correlations between SF RANTES and SF MIP-1α (P = 0·02), RANTES and IL-8 (P = 0·01), and MIP-1α and IL-8 (P < 0·01). The SF IL-8 levels were also significantly correlated with SF TNF-α (P < 0·01) and SF WBC counts (P < 0·01). Correlation analyses of SF Asp levels with SF TNF-α levels approached significance (P = 0·06, n = 44). Correlation analysis of SF Glu levels with SF WBC counts approached significance (P = 0·053, n = 40). Analyses of the SF inflammatory mediators were also conducted for each clinical disease and generally reflected the values obtained for the samples independent of disease (not shown). The SF sample numbers for individual diseases were not sufficient for detailed analysis.
Table 1.
Correlations of SF EAA, TNF-α, chemokine levels and white blood cell counts derived from patients with active arthropathies
| Asp | TNF-α | RANTES | MIP-1α | IL-8 | WBC† | |
|---|---|---|---|---|---|---|
| Glu | ||||||
| r2 = | 0·55 | 0·02 | 0·20 | 0·06 | 0·21 | 0·09 |
| P = | <0·01 | 0·31 | 0·03 | 0·09 | <0·01 | 0·053 |
| Asp | ||||||
| r2 = | 0·07 | 0·29 | 0·19 | 0·04 | 0·03 | |
| P = | 0·06 | <0·01 | <0·01 | 0·23 | 0·29 | |
| TNF-α | ||||||
| r2 = | 0·09 | 0·06 | 0·19 | 0·09 | ||
| P = | 0·16 | 0·11 | <0·01 | 0·06 | ||
| RANTES | ||||||
| r2 = | 0·24 | 0·25 | 0·01 | |||
| P = | 0·02 | 0·01 | 0·60 | |||
| MIP-1α | ||||||
| r2 = | 0·30 | 0·48 | ||||
| P = | <0·01 | 0·18 | ||||
| IL-8 | ||||||
| r2 = | 0·74 | |||||
| P = | <0·01 | |||||
The analyses were performed by the Pearson product-moment correlation. SF mediator concentrations were analysed independent of disease. TNF-α: tumour necrosis factor-alpha. Chemokines: RANTES (Regulated upon Activation Normally T-cell Expressed and Secreted), MIP-1α (macrophage inflammatory protein-1α) and IL-8 (Interleukin-8).
white blood cell count/mm3. The number of samples for each mediator correlation comparison ranged from 22 to 44.
Synovial cell culture TNF-α levels are increased with exogenous l-glutamate
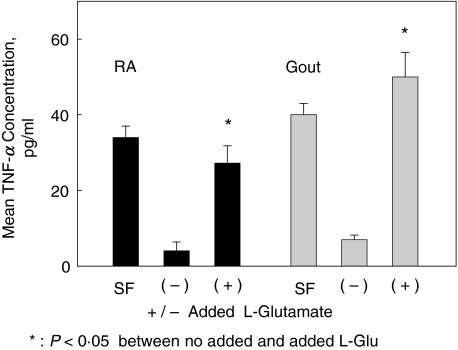
Figure 2 demonstrates increased TNF-α levels in supernatants of primary cultures from adherent cells exposed to added l-glutamate. At the time of synovial fluid harvest, the spun SF supernatants had mean TNF-α levels of 34·00 ± 3·2 pg/ml for the RA patient and 40·1 ± 3·7 pg/ml for the gout patient. After one week, mean TNF-α levels from culture supernatants were 4·02 ± 2·32 pg/ml for the RA cultures and 7 ± 1·2 pg/ml for the cultures from the acute gout patient (these baseline values are below the range for the TNF-α immunoassay). The cell cultures were incubated for two hours with (for gout) 250 µm or (for RA) 500 µm l-glutamate. This glutamate concentration range is consistent with the physiologic levels obtained in SF samples from RA, acute gout, Reiters and SLE patients [1]. Increased TNF-α concentrations were seen in the cell culture supernatants two hours after addition of l-glutamate. Two hour incubation with added l-glutamate resulted in a 6·77–7·14-fold increase in TNF-α levels, compared to the cultures that were incubated under similar conditions in the absence of added l-glutamate. After the l-glutamate containing media was removed, the cells were washed and replaced with normal culture media. The supernatant TNF-α levels had returned to baseline at 15 h after media replacement (not shown).
Fig. 2.
Mean TNF-α levels were determined in SF and from supernatants of primary synovial cells by immunoassay (ELISA). Mean TNF-α levels and standard deviations are expressed in pg/ml. SF: synovial fluid from arthrocentesis first pelleted and fluid aspirated to assess TNF-α levels. SF was also directly plated into culture and nonadherent cells were removed. After 7 days in culture, primary synovial cells were incubated with no added l-glutamate (–) or added L- glutamate (+) for two hours. Triplicate cultures were derived from one RA patient and one gout patient, on no specific anti-TNF-α therapy. Mean glutamate concentrations in the culture supernatants by HPLC were: no added l-glutamate: 50 µm, the same concentration as noted in the uncultured media; 500 µm added Glutamate; 470 µm, and 250 added l-glutamate: 230 µm. Cell cultures maintained viability by trypan blue exclusion at 2 and 15 h post glutamate incubation.
DISCUSSION
This study demonstrates high levels of SF EAA in patients with active RA, gout or OA, with variable SF cytokine and SF chemokine levels. In all arthropathies, the mean SF EAA, TNF-α and RANTES levels were significantly elevated, compared to the baseline levels obtained from nonarthritic, cadaver controls (not shown). Significant and strong correlations were noted between SF Glu and Asp levels and also among the SF chemokine levels. There were also significant, although less robust correlations between the SF Glu and SF RANTES or IL-8 levels and between SF Asp and SF RANTES or MIP-1α levels, independent of SF TNF-α levels. A previous study reported that treatment with selective anti TNF-α blocking agents resulted in a decrease in synovial MCP-1 and IL-8 levels, but not synovial RANTES or MIP-1α levels [17]. The correlations between Glu and RANTES and Asp and RANTES or IL-8 suggest that SF EAA's might provide an alternative mechanism to promote (or possibly sustain) SF chemokine up-regulation, independent of the status of SF TNF-α levels. Neurotransmitter up-regulation of SF chemokines might have important clinical relevance, as 20% of RA patients do not respond to anti-TNF-α therapy and over time, anti-TNF-α therapy may lose its efficacy [18,19]. More formal studies to assess the influence of TNF-α in synovial cultures will be necessary to assess if neurotransmitters Glu and Asp can directly up-regulate chemokines independent of TNF-α.
Previous studies have demonstrated 4·61-and 2·15-fold elevations of neurotransmitters Glu and Asp, respectively, relative to other amino acid concentrations, in active arthropathies [20]. In an experimental arthropathy, behavioural alterations and changes in joint circumference are seen as early as two hours post induction and correlated with an increase in intra-articular and spinal Glu concentrations [5,6,21,22]. Also, direct intra-articular injection of Glu results in an increase in joint blood flow, as measured by Doppler flow [23]. Other studies have shown Glu to be tightly regulated in culture in neuronally derived cells and our additional studies have demonstrated compartmental disparities of EAA in non-neuronal tissues [2,24]. Glu, leucine or isoleucine did not result in increased TNF-α [25] in cultured peripheral blood mononuclear cells, but this may reflect the tissue or compartment source. Glutamate receptor agonists resulted in increased TNF-α release in neuronal tissue, supposedly by microglial macrophages [26]. Other neurotransmitters have also been implicated in the development of inflammatory arthropathies [27–31]. Effective modulation of inflammatory and nociceptive parameters in arthritis have been studied with both peripheral and spinal cord manipulations [5,14,22,32–36].
The cell origins of the SF EAA, TNF-α or chemokine levels contributing to the active arthropathy can not be identified from the synovial fluids. The source of elevated SF EAA's might include leakage from nerve endings, generation by resident cells, reflecting direct production, or possibly compartmental disparity, reflecting transport or carrier protein influences. The cell origin for mediator synthesis or release might be dependent on the specific arthropathy [37–39]. Also, some mediators may be more prominent at early or later time points based on clinical activity [40,41]. These factors might confound direct assessment of inflammatory mediator relationships from synovial fluids
To further assess the relationship between SF Glu and TNF-α levels, primary synovial cell cultures derived from SF from RA and acute gout patients were set up in triplicate to assess the influence of added neurotransmitter Glu on TNF-α levels on synovial cultures. Incubation with l-glutamate demonstrated significantly increased TNF-α levels in the culture supernatants after two hours. It is unclear which cells (synoviocytes or macrophages) were responding to the l-glutamate, as both tissue macrophages and synoviocytes are capable of synthesizing TNF-α. In later experiments, established clonal fibroblast-like synoviocytes (SW892) also demonstrated increased TNF-α supernatant levels after exposure to glutamate receptor agonists, but usually as strong potentiators to other activators of TNF-α.
The culture data shows that neurotransmitter Glu and other glutamate receptor agonists can influence synovial cell TNF-α production, which in turn, can up-regulate additional cytokine and chemokine production and related mediators of inflammation. The above suggests two mechanisms by which SF neurotransmitters might promote inflammatory mediators, one that is TNF-α dependent and one that is TNF-α independent. Our lab has found glutamate receptors (NMDA and mGlu) on the cell surfaces of primary and established synoviocyte cultures. We have demonstrated that glutamate receptor activation increases cellular proteins (McNearney et al. unpublished observation).
This study provides evidence that synovial cells exposed to increased (pathological?) levels of Glu can trigger an inflammatory response, or possibly contribute to the maintenance of a sustained inflammatory response. This study also suggests that inflammatory mediators in active arthropathies can derive from several pathways and adjunct therapy may be necessary to truly contain many activators of synovial hypertrophy and inflammation.
Acknowledgments
The authors wish to thank Drs Shrilekha Sairam and Jharana Shrestha for their efforts in obtaining patient plasma and synovial fluids. Ms. Sue Stafford and Chrystel Cassan provided excellent technical assistance. The authors also thank Ms. Pat Gazzoli for her assistance in preparing the manuscript. This work was supported by grants from the NIH: NS 32118, Central Control of Arthritis and Arthritic Pain (KW) and NS11255 (KW,TM), Charles Dana Foundation (TM,KW) and Glaxo-Wellcome, Inc, Comparison of the Effects of Fluticasone and Montelukast on the Secretion of Cytokines and Chemokines in the Allergen-Challenged Nasal Mucosa (RA)
REFERENCES
- 1.McNearney T, Speegle D, Lawand N, Lisse J, Westlund KN. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol. 2000;27:739–45. [PMC free article] [PubMed] [Google Scholar]
- 2.McNearney T, Speegle D, Lawand N, Lisse J, Westlund K. Excitatory amino acid profiles of synovial fluids derived from patients with arthritis. Arthritis Rheum. 1999;42:S179. [PMC free article] [PubMed] [Google Scholar]
- 3.McNearney T, Goel N, Lisse J, Speegle D, Baethge B, Westlund KN. Temporal fluctuations in excitatory and inhibitory amino acid profiles of synovial fluids derived from patients with arthropathies. J Invest Med. 1999;47:109A. [Google Scholar]
- 4.McNearney T, Speegle D, Lawand N, Lisse J, Westlund K. Excitatory and inhibitory amino acid profiles of synovial fluids derived from patients with arthritis. J Investig Med. 1999;47:109A. [PMC free article] [PubMed] [Google Scholar]
- 5.Lawand NB, Willis WD, Westlund KN. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur. J Pharmacol. 1997;324:169–77. doi: 10.1016/s0014-2999(97)00072-1. [DOI] [PubMed] [Google Scholar]
- 6.Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint. key role in nociception and inflammation. Pain. 2000;86:69–74. doi: 10.1016/s0304-3959(99)00311-5. [DOI] [PubMed] [Google Scholar]
- 7.Hosaka S, Akahoshi T, Wada C, Kondo H. Expression of the chemokine superfamily in rheumatoid arthritis. Clin Exp Immunol. 1994;97:451–7. doi: 10.1111/j.1365-2249.1994.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szekanecz Z, Strieter RM, Kunkel SL, Koch AE. Chemokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:115–32. doi: 10.1007/BF00832002. [DOI] [PubMed] [Google Scholar]
- 9.Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol. 2001;167:5381–5. doi: 10.4049/jimmunol.167.9.5381. [DOI] [PubMed] [Google Scholar]
- 10.Young L, Katrib A, Cuello C, et al. Effects of intraarticular glucocorticoids on macrophage infiltration and mediators of joint damage in osteoarthritis synovial membranes: findings in a double-blind, placebo-controlled study. Arthritis Rheum. 2001;44:343–50. doi: 10.1002/1529-0131(200102)44:2<343::AID-ANR52>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Cutolo M, Sulli A, Barone A, Seriolo B, Accardo S. Macrophages, synovial tissue and rheumatoid arthritis. Clin Exp Rheumatol. 1993;11:331–9. [PubMed] [Google Scholar]
- 12.Baethge B, Forsythe PA, Alam R, Lisse JR. Macrophage inflammatory protein-1α (MIP-1α), RANTES and interleukin 8 (IL-8) in synovial fluids. FASEB J. 1994;8:A760. [Google Scholar]
- 13.Thornton S, Duwel LE, Boivin GP, Ma Y, Hirsch R. Association of the course of collagen-induced arthritis with distinct patterns of cytokine and chemokine messenger RNA expression. Arthritis Rheum. 1999;42:1109–18. doi: 10.1002/1529-0131(199906)42:6<1109::AID-ANR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Moore BA, Aznavoorian S, Engler JA, Windsor LJ. Induction of collagenase-3 (MMP-13) in rheumatoid arthritis synovial fibroblasts. Biochim Biophys Acta. 2000;1502:307–18. doi: 10.1016/s0925-4439(00)00056-9. [DOI] [PubMed] [Google Scholar]
- 15.Klippel JH. Primer on the Rheumatic Diseases. Atlanta: Arthritis Foundation; 2001. [Google Scholar]
- 16.Hachicha M, Rathanaswami P, Schall TJ, McColl SR. Production of monocyte chemotactic protein-1 in human type B synoviocytes. Synergistic effect of tumor necrosis factor alpha and interferon-gamma. Arthritis Rheum. 1993;36:26–34. doi: 10.1002/art.1780360106. [DOI] [PubMed] [Google Scholar]
- 17.Taylor PC, Peters AM, Paleolog E, et al. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:38–47. doi: 10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75) -Fc fusion protein. N Engl J Med. 1997;337:141–7. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 19.Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 20.McNearney T, Speegle D, Lawand N, Lisse J, Westlund KN. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J Rheumatol. 2000;27:739–45. [PMC free article] [PubMed] [Google Scholar]
- 21.Sluka KA, Westlund KN. An experimental arthritis in rats: dorsal horn aspartate and glutamate increases. Neurosci Lett. 1992;145:141–4. doi: 10.1016/0304-3940(92)90006-s. [DOI] [PubMed] [Google Scholar]
- 22.Sluka KA, Westlund KN. An experimental arthritis model in rats. the effects of NMDA and non-NMDA antagonists on aspartate and glutamate release in the dorsal horn. Neurosci Lett. 1993;149:99–102. doi: 10.1016/0304-3940(93)90357-q. [DOI] [PubMed] [Google Scholar]
- 23.Carlton SM, McNearney T, Cairns BE. The role of glutamate receptors in the periphery. Proceedings 10th World Conf Pain. 2003;24:125–39. [Google Scholar]
- 24.Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- 25.Rohde T, MacLean DA, Klarlund PB. Glutamine, lymphocyte proliferation and cytokine production. Scand. J Immunol. 1996;44:648–50. doi: 10.1046/j.1365-3083.1996.d01-352.x. [DOI] [PubMed] [Google Scholar]
- 26.de Bock F, Dornand J, Rondouin G. Release of TNF alpha in the rat hippocampus following epileptic seizures and excitotoxic neuronal damage. Neuroreport. 1996;7:1125–9. doi: 10.1097/00001756-199604260-00004. [DOI] [PubMed] [Google Scholar]
- 27.Lotz M, Carson DA, Vaughan JH. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science. 1987;235:893–5. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- 28.Colpaert FC, Donnerer J, Lembeck F. Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sci. 1983;32:1827–34. doi: 10.1016/0024-3205(83)90060-7. [DOI] [PubMed] [Google Scholar]
- 29.Marshall KW, Chiu B, Inman RD. Substance P and arthritis: analysis of plasma and synovial fluid levels. Arthritis Rheum. 1990;33:87–90. doi: 10.1002/art.1780330111. [DOI] [PubMed] [Google Scholar]
- 30.Bjurholm A, Kreicbergs A, Ahmed M, Schultzberg M. Noradrenergic and peptidergic nerves in the synovial membrane of the Sprague-Dawley rat. Arthritis Rheum. 1990;33:859–65. doi: 10.1002/art.1780330613. [DOI] [PubMed] [Google Scholar]
- 31.Cannon GW, Openshaw SJ, Hibbs JB, Jr, Hoidal JR, Huecksteadt TP, Griffiths MM. Nitric oxide production during adjuvant-induced and collagen-induced arthritis. Arthritis Rheum. 1996;39:1677–84. doi: 10.1002/art.1780391010. [DOI] [PubMed] [Google Scholar]
- 32.Lawand NB, McNearney T, Westlund KN. Amino acid release into the knee joint. key role in nociception and inflammation. Pain. 2000;86:69–74. doi: 10.1016/s0304-3959(99)00311-5. [DOI] [PubMed] [Google Scholar]
- 33.Boyle DL, Moore J, Yang L, Sorkin LS, Firestein GS. Spinal adenosine receptor activation inhibits inflammation and joint destruction in rat adjuvant-induced arthritis. Arthritis Rheum. 2002;46:3076–82. doi: 10.1002/art.10595. [DOI] [PubMed] [Google Scholar]
- 34.Sorkin LS, Moore J, Boyle DL, Yang L, Firestein GS. Regulation of peripheral inflammation by spinal adenosine: role of somatic afferent fibers. Exp Neurol. 2003;184:162–8. doi: 10.1016/s0014-4886(03)00102-x. [DOI] [PubMed] [Google Scholar]
- 35.Sorkin LS, Maruyama K, Boyle DL, Yang L, Marsala M, Firestein GS. Spinal adenosine agonist reduces c-fos and astrocyte activation in dorsal horn of rats with adjuvant-induced arthritis. Neurosci Lett. 2003;340:119–22. doi: 10.1016/s0304-3940(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 36.Schafers M, Marziniak M, Sorkin LS, Yaksh TL, Sommer C. Cyclooxygenase inhibition in nerve-injury- and TNF-induced hyperalgesia in the rat. Exp Neurol. 2004;185:160–8. doi: 10.1016/j.expneurol.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Matsukawa A, Yoshimura T, Maeda T, Takahashi T, Ohkawara S, Yoshinaga M. Analysis of the cytokine network among tumor necrosis factor alpha, interleukin-1beta, interleukin-8, and interleukin-1 receptor antagonist in monosodium urate crystal-induced rabbit arthritis. Laboratory Invest. 1998;78:559–69. [PubMed] [Google Scholar]
- 38.Matsukawa A, Yoshinaga M. Sequential generation of cytokines during the initiative phase of inflammation, with reference to neutrophils. Inflamm Res. 1998;47(Suppl. 3):S137–S144. doi: 10.1007/s000110050304. [DOI] [PubMed] [Google Scholar]
- 39.Smith RL, Trindade MCD, Ikenoue T, et al. Effects of shear stress on articular chondrocytes metabolism. Biorheaology. 2000;37:97–100. [PubMed] [Google Scholar]
- 40.Suzuki N, Nakajima A, Yoshino S, Matsushima K, Yagita H, Okumura K. Selective accumulation of CCR5+ T lymphocytes into inflamed joints of rheumatoid arthritis. Int Immunol. 1999;11:553–9. doi: 10.1093/intimm/11.4.553. [DOI] [PubMed] [Google Scholar]
- 41.Szekanecz Z, Halloran MM, Volin MV, et al. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant-induced arthritis. Arthritis Rheum. 2000;43:1266–77. doi: 10.1002/1529-0131(200006)43:6<1266::AID-ANR9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]