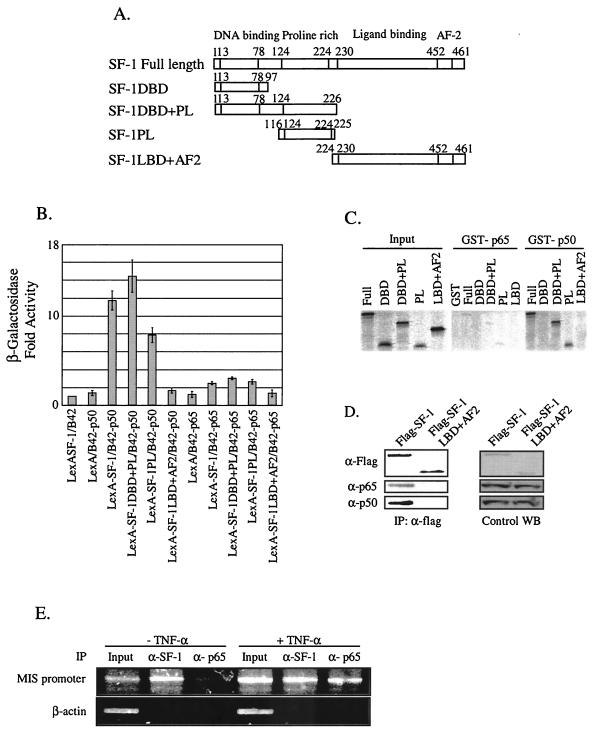
FIG. 4.
Association of NF-κB with SF-1 in vitro and in vivo. (A) Schematic diagrams of SF-1 full-length and deletion constructs. (B) The p50 subunit of NF-κB efficiently interacted with SF-1 via its proline-rich domain in yeast two-hybrid protein-binding assays. The interaction between SF-1 and either p65 or p50 was scored by the activation of β-galactosidase reporter. All values represent the mean ± the SEM of at least three independent colonies. (C) The p50 subunit directly interacted with SF-1 via its proline-rich domain in GST pull-down assays. [35S]methionine- labeled SF-1 and its deletion mutants were allowed to bind the GST fusion proteins of p65 and p50 subunits. Reactions were carried out with the equivalent amount of each protein, as determined by Coomassie blue staining (data not shown). Five percent of the labeled protein used in the binding reaction was loaded as input. (D) NF-κB was coimmunoprecipitated with SF-1. Flag-tagged full-length SF-1 expression plasmid or Flag-tagged SF-1LBD+AF2 was cotransfected with p65 and p50 expression plasmid into HeLa cells. Coimmunoprecipitations were conducted with anti-Flag antibody. Western blot analyses of immunoprecipitated materials were performed with anti-Flag, anti-p65, and anti-p50 antibody. Control Western blots (WB) are shown for the expression level of each protein. (E) NF-κB was recruited to the endogenous MIS promoter in the testis. ChIP assays were performed with organ-cultured 7-day-old testes treated or not treated with 10 ng of TNF-α/ml. Cross-linked DNA fragments were immunoprecipitated with anti-SF-1 or anti-p65 antibody, and the immunoprecipitates were analyzed by PCR with pairs of specific primers spanning the proximal promoter region of MIS. A control PCR for nonspecific immunoprecipitation was performed with primers specific to the β-actin coding region.