Abstract
The interaction of T cell CD28/CTLA-4 receptors with B7 on antigen-presenting cells (APCs) represents an important co-stimulatory pathway in T cell activation or anergy. Our previous study indicated that recipients immunized with allogenic donor immature dendritic cells (DCs) or resting B cells could induce specific immune tolerance and prolong allograft survival. A possible mechanism for this observation is that the expression of B7 molecules is either at a low level or lacking on these cells. The present study investigates whether blockade of B7 molecules on donor splenocytes with a B7 antisense peptide (B7AP), i.e. a peptide analogue of the CD28-binding region, could induce specific immune tolerance and prolong allograft survival in the recipients. Both the lymphocyte proliferation reaction and the mice pinna cardiac allograft experiment were performed to evaluate the role of B7AP in inducing specific immune tolerance in recipients in vitro and in vivo. The results showed that 56·65% and 20·52% of C57BL/6 splenocytes expressed B7.1 and B7.2 molecules, respectively, on their cell surface. There were no significant changes of the B7 expression on such splenocytes after being treated by the B7AP (53·28% and 19·06%, respectively). B7AP inhibited the mixed lymphocyte reaction by up to 38·4% and a dose-response correlation was observed for inhibition. The recipients (BALB/c) immunized with B7AP-pretreated C57BL/6 splenocytes induced a specific immune hypo-response (43%versus control) and notably prolonged survival of the C57BL/6 cardiac allograft by up to 20·3 days. In contrast to the normal saline group (average: 8·6 days) and FTD10 control peptide group (<4 days), the cardiac allograft survival of the test group was extended for an additional 11·7 days. These results strongly support the notion that immunization with donor splenocytes, which had been pretreated with B7AP, induced specific immune tolerance and prolonged allograft survival in the recipients.
Keywords: antisense peptide, B7, cardiac allograft, mixed lymphocyte reaction
INTRODUCTION
The B7–CD28/CTLA4 co-stimulatory pathway plays a crucial role in the regulation of T cell activation. Two B7 molecules, B7.1 (CD80) and B7.2 (CD86), are expressed on the surface of antigen-presenting cells (APCs) and provide a critical co-stimulatory signal to T cells by engaging CD28. B7 molecules can also provide a negative regulatory signal to T cells by binding to CTLA4 (CD152) [1–4]. Blockade of the B7–CD28 interaction in vitro can generate antigen-specific anergy [5–7]. Administration of monoclonal antibodies (MoAbs) against B7 or CTLA4–Ig fusion protein to block B7 has been shown to be promising as a treatment for allograft rejection [8–10]. Thus, the critical role of B7–CD28/CTLA4 interaction in determining the fate of immune responses (activation versus anergy) makes it an attractive target for therapeutic immunomodulations [11–13].
It has been reported that the recipients immunized with donor resting B cells or immature dendritic cells (DCs) could induce specific immune tolerance and prolong allograft survival. This effect has been attributed to low-level or no B7 molecule expression on these cells [14,15]. In recent years, there has been an increasing interest in the development of non-immunogenic peptide as an antagonist for protein–protein interaction in immunomodulatory therapeutics [16,17]. Progress in antisense technology and molecular modelling over the past decade has made molecular recognition study possible [18–20]. Antisense peptides are short peptide sequences that specifically constitute one side of the binding sites of complementary protein pairs [21]. In this study, we designed a mini peptide (termed B7AP) to block B7–CD28 interactions and tested the effect of its function on induction of allograft tolerance in vivo and in vitro.
MATERIALS AND METHODS
Synthesis and purification of the antisense peptide against B7
It has been reported that the MYPPPY motif is the core of the CD28 binding sites to its ligand B7. Several different peptides containing the motif were screened with the computer programs biopolymer and binding site analysis on the insight ii molecular modelling software package, and the B7AP was obtained with the sequence EFMYPPPYLD (data not shown). The peptide was synthesized on a solid phase peptide synthesizer (Multiple Peptide Synthesizer; Genemed Synthesis, Inc., CA, USA). The crude peptide was purified by the Varian Prostar high performance liquid chromatography (HPLC) system using a C8 column (Varian Prostar HPLC system, CA, USA). Analytical HPLC was performed through a Varian C8 analytical column using a linear gradient of 0–100% acetonitrile in water containing 0·1% trifluoroacetic acid over a period of 20 min. The identity of the peptide was confirmed by mass spectrometry (Voyager Elite model, Perseptive Biosystem, Applied Biosystems, WA, USA). The purity of the peptide (higher than 95%) was examined by HPLC analysis. Peptide was lyophilized and stored at −20°C until use.
Mice
Female BALB/c (H-2Kd), C57BL/6(H-2Kb) and C3H/HeJ (H-2Kk) mice (6–8 weeks old) were obtained from the Department of Laboratory Animal Science of Fudan University. Neonatal mice were also obtained from the same department and used within 24 h after birth.
FACS assay
C57BL/6 splenocytes were incubated for 30 min in PE-labelled antibody against H-2Kb (anti-H-2Kb–PE) or fluorescein isothiocyanate (FITC)-conjugated antimouse B7.1 and B7.2 antibody (anti-B7.1-FITC, anti-B7.2-FITC) and their own negative isotype control antibodies (mouse BALB/c IgG2, Armenian hamster IgG2, rat louvain IgG2a, respectively) (all the antibodies were purchased from PharMingen). The splenocytes were finally washed twice in Dulbecco's phosphate buffered saline (DPBS)/fetal calf serum (FCS) and analysed by FACS assay. The major histocompatibility complex (MHC) and B7 molecules were calculated from 10000 events collected.
Proliferation assay
Splenocytes were isolated from freshly harvested minced spleens of BALB/c or C57BL/6 mice by passing through a nylon mesh followed by lysis of the red blood cell. The C57BL/6 stimulator splenocytes were irradiated with 30 Gy γ−ray and washed once with phosphate buffered saline (PBS). The irradiated splenocytes were incubated with various concentrations of the B7AP at 37°C for 90 min. A total of 2·5 ×105 splenocytes were aliquoted into each well of a 96-well round-bottomed plate (Corning Glass Works, Corning, NY, USA). Freshly isolated responder BALB/c splenocytes were washed twice with serum-free RPMI, resuspended in complete RPMI medium and a total of 5 ×105 responder splenocytes in 100 µl volume were added into each well with stimulators. The cells were incubated for 5 days at 37°C and then pulsed with [3H]methylthymidine (0·5 µCi/well) during the last 18 h of culture. The cells were harvested onto glass fibre filters and assessed by liquid scintigraphy for thymidine incorporation. Triplicate samples were determined and the means of the results were expressed as counts per minute (cpm). The dose–response curves for the B7AP were consistently inhibitory in multiple experiments.
Induction of allo-hyporesponse in recipients
Single cell suspensions of donor C57BL/6 splenocytes (5 × 106/50 µl) in serum-free RPMI-1640 were irradiated with 30 Gy γ-ray and incubated with 7·8 µm B7AP at 37°C for 90 min. The treated cells were injected intravenously into 6–8-week-old BALB/c mice via the tail vein. Normal saline and C57BL/6 splenocytes without B7AP treatment were included as controls. Three days later, the splenocytes from the immunized BALB/c mice were collected as responder cells. Irradiated splenocytes from normal C57BL/6 and C3H/HeJ mice were used as stimulator cells. Mixed lymphocyte reaction (MLR) was performed as described above.
Mice pinna cardiac transplantation
Split-heart neonatal cardiac grafts were transplanted into the ear pinna of mice. Allografts from donors were placed in female recipients in a pinna location as described previously [22]. Graft function was assessed daily by anatomic microscopic observation and electrocardiographic monitoring. Rejection was defined as the absence of detectable electrocardiogram (ECG) of allograft. Allograft failures or death of recipients within 48 h of surgery were considered technical failures and were excluded from the analysis.
Statistical analysis
The statistical analysis was performed using the spss 10·0 software package. For in vitro proliferation assay, Tukey's test in the one-way anova module was used to determine the statistical significance of cpm, and the log-rank test was performed to analyse the survival time of cardiac allograft among groups. Quadratic equation was used to describe the dose–response relationship between cpm and the concentration of B7AP.
RESULTS
Expression of B7 and MHC-I on C57BL/6 mice splenocytes
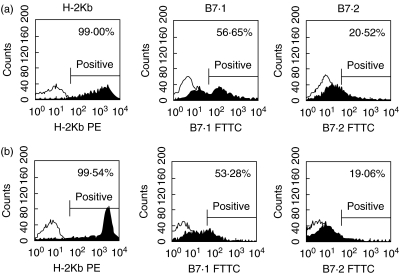
Flow cytometric analysis revealed that the MHC-I (H-2Kb) molecule is expressed on the cell surface of 99% of splenocytes of C57BL/6 mice. By contrast, the results showed that 56·65% and 20·52% of C57BL/6 splenocytes expressed B7.1 and B7.2 molecules respectively on their cell surface. There were no significant changes in B7 expression on the splenocytes after treatment with B7AP (53·28% and 19·06%, respectively) (Fig. 1).
Fig. 1.
Expression of B7 and MHC-I (H-2Kb) molecules on C57BL/6 splenocytes. (a) C57BL/6 splenocytes; (b) C57BL/6 splenocytes after B7AP treating. Freshly prepared C57BL/6 spleen cells were stained with either anti-H-2Kb-PE, anti-B7.1-FITC or anti-B7.2-FITC (a). The irradiated C57BL/6 spleen cells were treated with B7AP as above and then the expression of B7 and MHC-I (H-2Kb) molecules was detected in the same way. The expression of B7 or MHC-I by the indicated cell fractions is represented by filled histograms. The open histograms represent control staining with an isotype control antibody. One representative of three independent experiments is shown.
Inhibition of MLR by B7AP
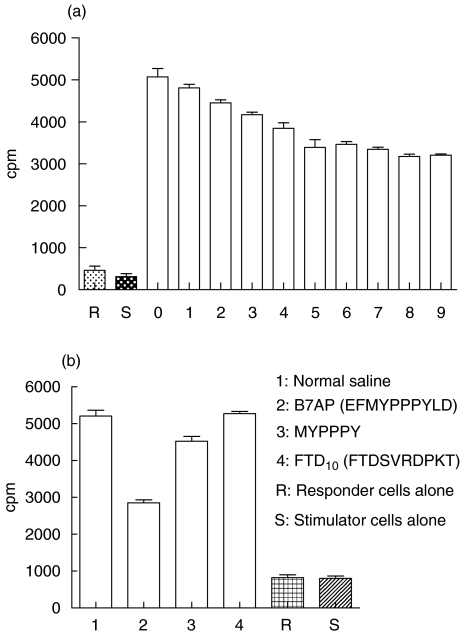
Based on our molecular modelling results and previous studies [23], we have identified a short peptide that corresponds to the B7-binding site of CD28, EFMYPPPYLD, as a potential B7AP. It was predicted to bind B7 molecules when used as a soluble peptide. Hence, we synthesized and purified this antisense peptide (Materials and methods) and tested whether it could indeed block CD28–B7 interaction and measured its effect on T cell activation. We first performed a mixed lymphocyte reaction to determine whether this peptide could inhibit CD28–B7 interaction in vitro. As shown in Fig. 2, BALB/c T cell proliferation was significantly inhibited when stimulator C57BL/6 splenocytes were treated with B7AP. The inhibitory effect of B7AP was 37·4% compared to the positive control. When the concentrations of B7AP rose to 7·8 µm, the B7AP did not provide significant additional inhibition in the mice MLR. For example, the percentage of inhibition was 38·4% at 78 µm. Therefore, 7·8 µm was chosen to be the working concentration in the following experiments. To test the specificity of the inhibitory effect of B7AP, a control peptide (FTD10) was synthesized and the MLR was performed at the same concentration of the peptides (7·8 µm). The result is shown in Fig. 2b. All groups have significant differences compared to the B7AP group. The inhibitory effect of MYPPPY was only 13·2% and the FTD10 control groups showed no significant difference compared to the normal saline (NS) group (t-test P > 0·05). The data suggested that the inhibitory effect of B7AP was specific (MYPPPY: relative peptide; FTD10 control group: FTDSVRDPKT).
Fig. 2.
B7AP inhibited the mixed lymphocyte reaction (MLR). Irradiated C57BL/6 splenocytes (stimulators) were pretreated with B7AP of serial dilutions. Stimulator cells (2·5 × 105) were combined with responder cells (BALB/c splenocytes) (5 × 105) in the absence or presence of B7AP and tested in MLRs. Cells were incubated for 5 days at 37°C and pulsed with [3H]methylthymidine (0·5 µCi/well) for the last 18 h. Data are representative of nine different concentrations. Experiments were performed in triplicate cultures. Final peptide concentrations are indicated on the x-axis; numbers 1–9 represent 7·8 × 10−6, 7·8 × 10−5, 7·8 × 10−4, 7·8 × 10−3, 7·8 × 10−2, 7·8 × 10−1, 7·8, 78, 156 µm, respectively; y-axis indicates proliferation of the responder cells in cpm. R refers to responder cells alone, S refers to stimulator cells alone and 0 refers to the group whose stimulator cells had not been pretreated with the B7AP. The irradiated C57BL/6 splenocytes (stimulators) were pretreated by the B7AP and control peptide at the same concentration (7·8 µm), and the MLR was performed as described above (Fig. 2b). NS: normal saline group, MYPPPY group (relative peptide), negative control group: FTD10 (FTDSVRDPKT).
Induction of allo-hyporesponse by donor splenocytes pretreated with B7AP
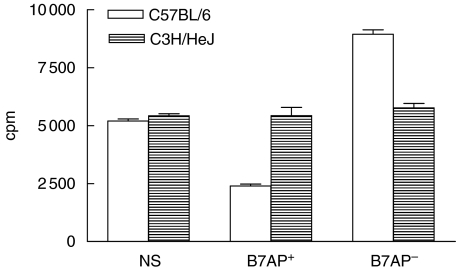
In order to test whether the recipient (BALB/c) could be induced to have reduced allo-responses after immunization with donor (C57BL/6) splenocytes pretreated with B7AP, three days after immunization with the pretreated C57BL/6 splenocytes, the splenocytes of the immunized BALB/c mice were obtained (as responder cells) and cultured with irradiated C57BL/6 or C3H/HeJ splenocytes as stimulator cells. On day 5, proliferation of BALB/c responder splenocytes was significantly decreased with C57BL/6 splenocyte stimulation. The average percentage of inhibition by C57BL/6 splenocytes stimulation was 43%versus controls (Fig. 3). Interestingly, the proliferation of the responder cells was not affected when they were stimulated with C3H/HeJ splenocytes. Based on the results, it was concluded that an allo-hyporesponse could be achieved with B7AP in vivo.
Fig. 3.
Immunization with donor splenocytes, which had been pretreated with B7AP induced allograft hyporesponsiveness. Responder cells refer to the BALB/c splenocytes that had been preimmunized with the following materials 3 days before: NS, normal saline (negative control); B7AP– group, irradiated C57BL/6 splenocytes (positive control); B7AP+ group, irradiated C57BL/6 splenocytes pretreated with B7AP (experimental group). Stimulator cells refer to irradiated C57BL/6 and C3H/HeJ splenocytes. Stimulator cells (2·5 × 105) were mixed with responder cells (5 × 105) and tested in MLRs as described in Materials and methods. Cells were incubated for 5 days at 37°C and pulsed with [3H]methyl-thymidine (0·5 µCi/well) for the last 18 h.
Prolongation of allograft survival induced by donor splenocytes pretreated with B7AP
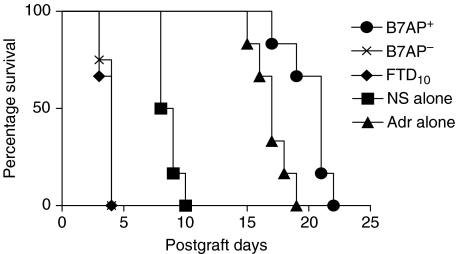
We next tried to determine whether immunizing the recipient (BALB/c) with donor (C57BL/6) splenocytes pretreated with the B7AP could prolong allograft survival. On the third day after immunization with the B7AP pretreated splenocytes, the split cardiac allografts obtained from neonatal C57BL/6 mice were transplanted into the ear pinna of the preimmunized BALB/c mice. C57BL/6 splenocytes treated with adriblastine group (positive control), FTD10 group (negative control) and untreated C57BL/6 splenocytes (B7AP–) (self-control) were included in the experiment as controls. As shown in Fig. 4, cardiac allograft survival in recipients immunized with donor splenocytes pretreated with B7AP was up to 20·3 days. The maximal survival period was 22 days. Compared to the normal saline group (average = 8·6 days), the survival of cardiac allograft of the B7AP group was extended for an additional 11·7 days (log-rank test, P < 0·001, n = 6). The survival times in the adriblastine, the C57BL/6 splenocytes and the FTD10 groups were 17 days, <4 days and <4 days, respectively, which was consistent with previous reports [24]. These results indicated that immunizing recipients with donor splenocytes pretreated with B7AP could prolong the survival of allograft.
Fig. 4.
Immunization with donor splenocytes preblocked with B7AP induced prolonged allograft survival. Single cell suspension of donor C57BL/6 spleen cells (5 × 106/50µl) in serum-free RPMI-1640 were irradiated with 30 Gy gamma-ray and precultured with 7·8µm B7AP at 37°C for 90min. These cells were then injected i.v. via the tail vein into 6–8-week-old BALB/c mice: B7AP+ (•). The controls were the normal saline group: NS (▪), adriblastine group: Adr (▴), negative control FTD10 group (♦) and untreated C57BL/6 splenocytes: B7AP– (×). Three days later, neonatal C57BL/6 split-heart cardiac grafts were transplanted into the ear pinna of these preimmunized BALB/c mice. Graft survival was assessed daily by anatomic microscope observation and electrocardiographic monitoring with rejection defined as the absence of a detectable beat.
DISCUSSION
Besides the interaction of the TCR and MHC-antigen complex, a productive immune response and maintainance of T cell homeostasis is determined largely by co-stimulation provided by the interacting B7–CD28/CTLA4 molecules [25]. It is generally believed that B7–CD28 interaction up-regulates T cell activation, whereas B7–CTLA4 interaction plays an inhibitory role in T cell activation. A unique feature of the B7–CD28/CTLA4 interaction that can be exploited for selective blockade of CD28 signalling is the differential kinetics of binding with CTLA4, exhibiting a faster on–off rate and higher affinity for B7 ligands than CD28 [26]. It was also reported that CTLA4 binds B7.1 and B7.2 with >500-fold higher avidity than does CD28 [27].
Previous studies targeting the B7–CD28/CTLA4 pathway for immunotherapeutic modulation of T cell responses have produced variable outcomes [28–30]. Treatment with CTLA4-Ig, a fusion protein that blocks both B7.1 and B7.2, has been shown to either alleviate or exacerbate the clinical signs of EAE, depending on the timing of administration [31,32]. These opposing results have been attributed to unintended simultaneous inhibition of B7–CTLA4 interaction, which down-regulates T cell activation. Therefore, selective blockade of B7–CD28 interaction alone while sparing the B7–CTLA4 interaction would be an attractive approach for achieving intended maximal inhibitory effects or therapeutic modulations.
In the present study, we hypothesized that an analogue of the ligand-binding region of CD28 could selectively block B7–CD28 interactions by blocking CD28 for binding to B7 ligands. By contrast, CTLA4 could theoretically overcome this competition due to its higher affinity for B7 ligands. Based on previous reports and our results of molecular modelling using the insight ii molecular modelling software package, we have chosen EFMYPPPYLD as the B7AP. The expression of MHC-I, B7.1 and B7.2 molecules on C57BL/6 splenocytes were 99·00%, 56·65% and 20·52%, respectively (Fig. 1). The spleen cells showed high levels of MHC-I molecules, and the expression of B7 and especially B7.1 within this cell population. By contrast, the B7.2 was at a relatively low level and was only 20·52%. To test whether, instead, loading of the B7 molecules with the peptide is simply causing increased internalization and therefore reduced surface levels of protein, Fig. 1 also shows that there are no significant changes of B7 molecule expression after B7AP treatment.
The results in Fig. 2a show that the B7AP could block the expansion of T cells in MLRs in a dose-dependent manner. The result suggested that the B7AP could bind efficiently to B7, block the interaction of CD28–B7 and reduce the allo-response in vitro. The specificity of the inhibitory effect of B7AP was shown in Fig. 2b. The degree of inhibition of MLR could be up to 37·4% (7·8 µm) in our experiment. Collectively, Fig. 2a manifested that the B7AP could partially suppress B7–CD28 interactions even if the concentration of the peptide is increased in the culture system. This suggests that, in addition to B7–CD28 interaction, other co-stimulatory pathways such as CD40–CD40L could also contribute to this process [33]. It is conceivable that competition between B7AP and CD28 for binding to the B7 ligands may result in a quantitative reduction in CD28 receptor occupancy, therefore decreasing the probability of CD28 signalling. This is consistent with previous observations that a reduction in the expansion of allo-reactive T cells was detected following blockade of CD28 by anti-CD80 antibody or CTLA4-Ig.
Our laboratory has reported previously that immunization with donor resting B cells or immature DCs could induce specific immune tolerance and prolong allograft survival. An increasing body of literature supports the possible mechanism that induced immune tolerance is due to the low-level or no B7 expression on resting B cells and immature DCs. Immunization with these cells induced a specific allograft tolerance [34]. Because the B7AP derived from the ligand-binding region of CD28 could block B7 on APCs, we also addressed the question of whether immunizing the recipient with allogenic donor splenocytes pretreated with B7AP could also induce allograft tolerance, as observed with resting B cells and immature DCs. Splenocytes from mice immunized with allogenic donor splenocytes pretreated with B7AP showed a reduced allo-response in vitro (Fig. 3). The level of allo-specific inhibition was 43% compared to control group. Furthermore, in parallel cardiac allograft experiments, the survival period of cardiac allograft was 11·6 days longer compared with the self-control group (n = 6, P < 0·001) (Fig. 3). Thus, immunization with allogenic donor splenocytes pretreated with B7AP could not only induce specific immune tolerance but also prolong cardiac allograft survival in the recipients.
Current strategies for immunomodulation of allo-reactive T cell responses include the use of monoclonal antibodies to block the critical molecules in T cell activation including B7–CD28. However, as effective therapeutic agents, antibodies are limited by virtue of their inherent immunogenicity. In contrast, small peptide-based therapy is less likely to be immunogenic and can be used over a longer period of time [35]. Furthermore, peptides have substantially lower molecular weight and can cross tissue barriers into the target organ such as the kidney more easily. Because the donor splenocytes can be obtained more readily than the donor resting B cells and immature DCs, which require time-consuming culture, the strategy of treating donor splenocytes with B7AP can potentially be used in clinical transplantation. In addition to providing a novel approach to developing new therapeutic agents, bioactive peptides designed to mimic the surface epitopes involved in protein–protein interaction offers a powerful tool for characterizing the mechanisms involved in immune responses.
Acknowledgments
We thank Wei Xu, Huanbing Xu, Liping Su, Xianan Shao and Bing Qiao for helpful discussions. We thank Qingdong Guan and Xiujuan Zheng for assistance with animal experiment. This study was supported by the Major Program of Shanghai Municipal Natural Science funds (03JC14085), the Major State Basic Research Development Program of People's Republic of China (2004AA215242) and National Natural Science Funds of China (no. 39830340).
REFERENCES
- 1.Alegre M, Fallarino F, Zhou P, et al. Transplantation and the CD28/CTLA4/B7 pathway. Transplant Proc. 2001;33:209–311. doi: 10.1016/s0041-1345(00)01977-1. [DOI] [PubMed] [Google Scholar]
- 2.Kearney ER, Walunas TL, Karr RW, Morton PA, Oh DY, Bluestone JA. Antigen-specific CD4+ T cells in vivo is dependent on CD28 co-stimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–6. [PubMed] [Google Scholar]
- 3.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 4.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–56. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 6.Arpinati M, Terragna C, Chirumbolo G, et al. Human CD34(+) blood cells induce T-cell unresponsiveness to specific alloantigens only under co-stimulatory blockade. Exp Hematol. 2003;31:31–8. doi: 10.1016/s0301-472x(02)01018-4. [DOI] [PubMed] [Google Scholar]
- 7.Jonker M, Ossevoort AM, Vierboom M. Blocking the CD80 and CD86 co-stimulation molecules: lessons to be learned from animal models. Transplantation. 2002;73(Suppl. 1):S23–6. doi: 10.1097/00007890-200201151-00009. [DOI] [PubMed] [Google Scholar]
- 8.Najafian N, Sayegh MH. CTLA4-Ig: a novel immunosuppressive agent. Exp Opin Invest Drugs. 2000;9:2147–57. doi: 10.1517/13543784.9.9.2147. [DOI] [PubMed] [Google Scholar]
- 9.Woodward JE, Salam A, Logar AJ, Schaefer AT, Rao AS. Flt3-L augments the engraftment of donor-derived bone marrow cells when combined with sublethal irradiation and co-stimulatory (CD28/B7 and CD40/CD40L) blockade. Cell Transplant. 2002;11:147–59. [PubMed] [Google Scholar]
- 10.Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. CD28-specific antibody prevents graft-versus-host disease in mice. J Immunol. 2000;164:4564–8. doi: 10.4049/jimmunol.164.9.4564. [DOI] [PubMed] [Google Scholar]
- 11.Karandikar NJ, Vanderlugt CL, Bluestone JA, Miller SD. Targeting the B7:CD28/CTLA4 co-stimulatory system in CNS autoimmune disease. J Neuroimmunol. 1998;89:10–8. doi: 10.1016/s0165-5728(98)00058-7. [DOI] [PubMed] [Google Scholar]
- 12.Kurlberg G, Haglind E, Schon K, Tornqvist H, Lycke N. Blockade of the B7–CD28 pathway by CTLA4-Ig counteracts rejection and prolongs survival in small bowel transplantation. Scand J Immunol. 2000;51:224–30. doi: 10.1046/j.1365-3083.2000.00725.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka J, Asaka M, Imamura M. T-cell co-signalling molecules in graft-versus-host disease. Ann Hematol. 2000;79:283–90. doi: 10.1007/s002779900134. [DOI] [PubMed] [Google Scholar]
- 14.Yang XL, Ye WF, He QZ. Experimental study on in vitro of allo-hyporesponsiveness by resting cells. Shanghai J Immunol. 2001;21:80–3. [Google Scholar]
- 15.Ye WF, He QZ. Experimental study on induction of allo-hypo-immuno-responsiveness by immature DCs. Chinese J Immunol. 2001;16:578–81. [Google Scholar]
- 16.Clackson T, Wells JA. A hot spot of binding energy in a hormone–receptor interface. Science. 1995;267:383–6. doi: 10.1126/science.7529940. [DOI] [PubMed] [Google Scholar]
- 17.Wrighton NC, Farrell FX, Chang R, et al. Small peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273:458–63. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 18.Bajorath J, Metzler WJ, Linsley PS. Molecular modeling of CD28 and three-dimensional analysis of residue conservation in the CD28/Cd152 family. J Mol Graph Model. 1997;15:135–9. doi: 10.1016/S1093-3263(97)00020-X. [DOI] [PubMed] [Google Scholar]
- 19.Conte LL, Chothia C, Janin J. The atomic structure of protein–protein recognition sites. J Mol Biol. 1999;285:2177–98. doi: 10.1006/jmbi.1998.2439. [DOI] [PubMed] [Google Scholar]
- 20.Sayegh MH, Turka LA. The role of T-cell co-stimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–21. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 21.Slootstra JW, Roubos EW. Sense-antisense complementarity in protein–protein interaction sites. In: Joseph NMM, Alexander RK, editors. Antisense nucleic acids and proteins: fundamentals and applications. New York: Marcel Dekker, Inc.; 1991. pp. 205–28. [Google Scholar]
- 22.Fey TA, Krause RA, Hsieh GC, et al. Improved methods for transplanting split-heart neonatal cardiac grafts into the ear pinna of mice and rats. J Pharmacol Toxicol Meth. 1998;39:9–17. doi: 10.1016/s1056-8719(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan M, Wardrop RM, Gienapp IE, Stuckman SS, Whitacre CC, Kaumaya PTP. A retro-inverso peptide mimix of CD28 encompassing the MYPPPY motif adopts a polyproline type II helix and inhibits encephalitogenic T cells in vitro. J Immunol. 2001;167:578–85. doi: 10.4049/jimmunol.167.1.578. [DOI] [PubMed] [Google Scholar]
- 24.Yang XL, Ye WF, He QZ. Prolongation of cardiac allograft survival by immunization with donor resting B cells in mice. Shanghai J Immunol. 2001;21:154–7. [Google Scholar]
- 25.Suresh M, Whitmire JK, Harrington LE, et al. Role of CD28–B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–73. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 26.Mewrwe PA, Bodian DL, Daenke S, Linsley P, Davis SJ. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–400. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE. Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem. 1996;271:30375–80. doi: 10.1074/jbc.271.48.30375. [DOI] [PubMed] [Google Scholar]
- 28.Anderson DE, Sharpe AH, Hafler DA. The B7–CD28/CTLA4 co-stimulatory pathways in autoimmune disease of the central nervous system. Curr Opin Immunol. 1999;11:677–83. doi: 10.1016/s0952-7915(99)00036-9. [DOI] [PubMed] [Google Scholar]
- 29.Benhamou PY. Immunomodulation with CTLA4-Ig in islet transplantation. Transplantation. 2002;73:540–2. doi: 10.1097/00007890-200201151-00013. [DOI] [PubMed] [Google Scholar]
- 30.Khoury SJ, Akalin E, Chandraker A, et al. CD28–B7 co-stimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155:4521–4. [PubMed] [Google Scholar]
- 31.Chitnis T, Najafian N, Abdallah KA, et al. CD28-independent induction of experimental autoimmune encephalomyelitis. J Clin Invest. 2001;107:575–83. doi: 10.1172/JCI11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–7. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 33.Yamada AA, Sayegh MH. The CD154–CD40 co-stimulatory pathway in transplantation. Transplantation. 2002;73(Suppl. 1):S36–9. doi: 10.1097/00007890-200201151-00012. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa K, Matsuno T, Iwagaki H, et al. Donor dendritic cells and recipient Kupffer cells in the induction of donor-specific immune hyporesponsiveness. J Int Med Res. 2001;29:119–30. doi: 10.1177/147323000102900209. [DOI] [PubMed] [Google Scholar]
- 35.Erbe DV, Wang S, Xing Y, Tobin JF. Small molecule ligands define a binding site on the immune regulatory protein B7.1. J Biol Chem. 2002;277:7363–8. doi: 10.1074/jbc.M110162200. [DOI] [PubMed] [Google Scholar]