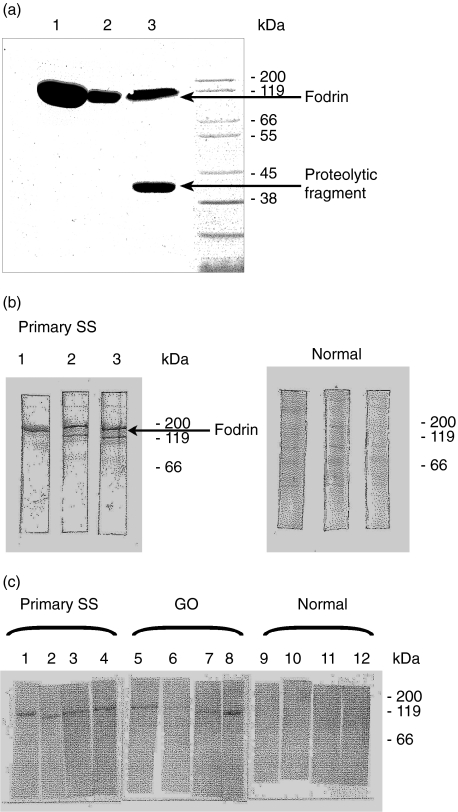
Fig. 1.
(a) Enhanced expression of recombinant human α-fodrin in BL21-CodonPlus™ cells: an aliquot of either BL21-CodonPlus™ (DE3)-RIL cells (lane 1 or far left) or BL21-Gold (DE3) cells (median lane 2), or E. coli M15 cells (right lane) expressing the recombinant human fodrin gene, was induced at mid-logarithmic growth with 1 mm IPTG for 2 h. After centrifugation each culture was homogenized in binding buffer, cleared by centrifugation and loaded on a Ni-NTA-resin. The eluted proteins were separated on a 10% acrylamide gel and stained with Coomassie® blue. (b) Immunoblot analysis with the recombinant alpha fodrin from experiment (a). Equal quantities of protein from the small scale Ni-NTA-purification of the above experiment were separated by electrophoresis, transferred to nitrocellulose membranes und probed with a 1: 200 dilution of a serum sample from a patient with a primary Sjögren syndrome or a serum sample from a human healthy volunteer. (c) Reactivities to recombinant alpha fodrin in GO sera. Purified protein was fractionated and transferred as in experiment (b) and probed with sera from the indicated humans.