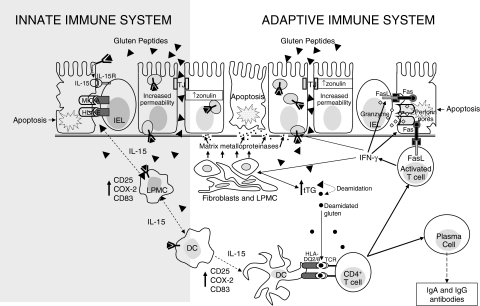
Fig. 1.
Epithelial transport and immune recognition of gluten in coeliac disease. In coeliac disease gluten peptides may cross the intestinal epithelium either via the paracellular route or via the transcellular route. In the first case this occurs as a consequence of an increased permeability due to an up-regulation of zonulin (which might be gliadin-dependent or gliadin-independent), in the second case it occurs by using enterocytic vesicles carrying the HLA class II-peptides complex. These vesicles are able to cross the basal membrane allowing intact gluten peptides to have access to the lamina propria. Here, they can activate both dendritic cells and lamina propria mononuclear cells to produce interleukin 15 which in turn causes an up-regulation of stress protein by enterocytes that are recognized by the natural killer receptors present on intraepithelial lymphocytes. On the other hand, native and tTG-deamidated gluten peptides are presented by mature dendritic cells to T cell receptors by using the heterodimer encoded by HLA-DQ2 or DQ8 genes A and B. This bond strongly activates CD4+ T cells which, by secreting pro-inflammatory cytokines, stimulate cytotoxic T cells and fibroblasts to produce a particular matrix metalloproteinase pattern which is responsible for degradation of both extracellular matrix and basement membrane. Activated T cells also become able to trigger enterocyte apoptosis by producing molecules like Fas ligand and granzyme, which are responsible for cytotoxicity, leading to the characteristic mucosal lesions. Stimulated CD4+ T cells are also able to induce lymphocyte B differentiation into plasma cells producing specific antigladin and anti-tissue transglutaminase antibodies. DC, dendritic cell; FasL, Fas ligand; HLA, human leucocyte antigen; IL, interleukin; IEL, intraepithelial lymphocyte; LPMC, lamina propria mononuclear cell; TCR, T cell receptor; TJ, tight junction; tTG, tissue transglutaminase.