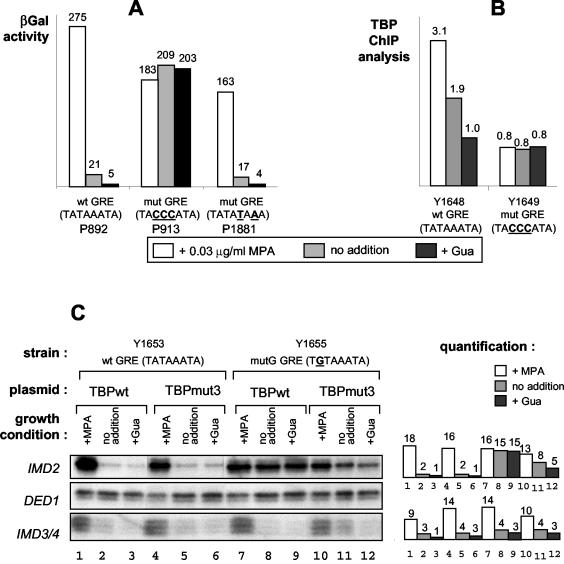
FIG. 5.
The GRE is the IMD2 TATA box. (A) Plasmids carrying IMD2-lacZ fusions with the indicated GRE sequences (wt, wild type; mut, mutant) were transformed into wild-type Y350 cells that were treated or not treated with guanine (Gua) or MPA. β-Galactosidase (βGal) activity was then measured. (B) The TBP occupancy of the IMD2 promoter was measured by ChIP analysis with isogenic strains carrying either the wild-type or the mutant GRE and treated or not treated with Gua or MPA. The amount of IMD2 promoter chromatin in the control or TBP immunoprecipitates was normalized to that of input DNA, and the indicated values are the relative levels in TBP versus control immunoprecipitates. An analysis of internal controls, showing no change in TBP occupancy, was also performed by PCR amplification of both the ACT1 TATA box region and a non-TATA box-containing region of the IMD2 gene corresponding to its coding sequence (data not shown). (C) A mutant TBP recognizing a TGTAAATA mutant TATA box (mutation in boldface) is able to restore MPA and Gua regulation to a strain with a TGTAAATA mutant GRE box at the IMD2 locus. The indicated strains were transformed with a multicopy plasmid expressing wild-type TBP (TBPwt) or an altered-specificity mutant of TBP (TBPmut3) that can bind the TGTAAATA sequence. Total cellular RNA was extracted from cells grown in the presence or absence of Gua and MPA, and the levels of transcripts of IMD2 and DED1 (as an internal control) were measured by S1 nuclease analysis. A portion of the IMD2 probe can hybridize to the IMD3 and/or IMD4 transcripts, and this hybridization is revealed by a very small labeled DNA fragment protected from S1 nuclease digestion. We included this hybridization product in the figure, since it actually demonstrates that activation by MPA was similar in the different strains. Quantification of the blots (arbitrary units) is shown on the right.