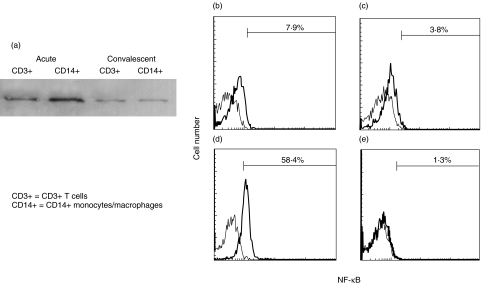
Fig. 1.
NF-κB activation in peripheral blood CD3+ T cells and CD14 + monocytes/macrophages of a 2-month-old boy with KD. (a) Nuclear extracts were harvested from CD14+ monocytes/macrophages or CD3+ T cells. The nuclear extracts were used as the sample for Western blotting because activated NF-κB existed in the nucleus. Rabbit polyclonal antibodies against NF-κB-p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as the primary antibodies.Western blot analysis demonstrated that intranuclear amount of NF-κB was increased in CD14 + monocytes/macrophages and CD3+ T cells at the acute stage compared with that at the convalescent stage. (b–e) Whole blood was labelled with phycoerythrin-conjugated with anti-CD14 moclonal antibodies and peridinin chlorophyll protein-conjugated anti-CD3 monoclonal antibodies and then permeabilized in 4% paraformaldehyde in phosphate-buffered saline, pH 7·2, containing 0·1% saponin and 10 mm HEPES. The cells were then labelled with a mouse anti-NF-κB (nuclear-localized signal) antibody (IgG3; Boehringer Mannheim, Mannheim, Germany). The mouse anti-NF-κB (nuclear-localized signal) antibody recognizes an epitope overlapping the nuclear location signal of NF-κB-p65 and therefore selectively recognizes the activated form of NF-κB. The cells were then labelled with a FITC-conjugated rat antimouse IgG3 monoclonal antibody (Pharmingen, San Diego, CA, USA). Immunofluorescence was analysed with a FACScan flow cytometer equipped with CellQuest software (Becton-Dickinson Biosciences, San Jose, CA, USA). The percentages of cells with intranuclear NF-κB in CD14+ monocytes/macrophages and CD3+ T cells by flow cytometric analysis are indicated. (b) CD3+ T cells at the acute stage; (c) CD3+ T cells at the convalescent stage; (d) CD14 + monocytes/macrophages at the acute stage; (e) CD14 + monocytes/macrophages at the convalescent stage.