Abstract
This study was undertaken to evaluate the possible role of hepatitis B recombinant vaccine inducing the synthesis of IgG and IgM anti-cardiolipin antibodies (aCL), antibodies against β2GPI (anti-β2GPI), lupus anti-coagulant (LA), anti-nuclear antibodies and antibodies against extractable nuclear antigens (anti-ENA). The study population consisted of 85 healthy students (63 female, 22 male; mean age 20·8 years), vaccinated with three doses of recombinant DNA hepatitis B vaccine. One month after vaccination with the first dose of hepatitis B vaccine a minority of vaccinated individuals showed changes in IgG or IgM aCL or anti-β2GPI or LA activity (P < 0·001). Among subjects in whom changes of IgG anti-β2GPI were observed, a significantly higher number of increased (8/85) than decreased (2/85) values were found (P < 0·01). Analyses of paired data showed that differences in aCL or anti-β2GPI levels before vaccination or 1 month later did not reach statistical significance. In two people aCL transitorily reached medium positivity after the first dose of hepatitis B vaccine with a drop 5 months later. Similar evident anti-β2GPI fluctuation was also observed in one person. Another participant was initially low positive for IgG anti-β2GPI and the levels were increasing after vaccination. Two participants became positive for anti-nuclear antibodies during 6 months' follow-up. There were no sex-dependent differences in tested antibodies observed and no associations between levels of aPL and levels of anti-HBV antibodies. We conclude that HBV can induce aPL, although rarely. In genetically susceptible individuals or together with some other triggers such combination might confer the risk of developing a continuous autoimmune response in an individual.
Keywords: autoimmunity, hepatitis B, vaccination
Introduction
Anti-phospholipid antibodies (aPL) are a heterogeneous family of antibodies that react to negatively charged phospholipids or phospholipid–protein complexes. The association between aPL and autoimmune diseases has long been recognized, but more recently it has also been suggested that infections may be a trigger for the induction of pathogenic aPL.
Two groups have supplied experimental data to support the infective aetiology of anti-phospholipid syndrome (APS), establishing a mechanism of molecular mimicry in experimental APS. Blank et al. identified a hexapeptide (TLRVYK) that is specifically recognized by a pathogenic anti-β2 glycoprotein I (anti-β2GPI) monoclonal antibodies and has a high homology with peptidic domain of various bacteria and viruses [1]. In addition, Gharavi et al. demonstrated induction of pathogenic aPL in mice by immunization with cytomegalovirus-derived synthetic peptides that share structural similarity with the putative phospholipid-binding region of the β2GPI molecule [2].
In recent years, medical and public interest in the safety of vaccination has been heightened by reports of possible vaccine-induced autoimmune disorders, in particular those related to hepatitis B vaccine [3]. This study was undertaken to evaluate the possible role of hepatitis B recombinant vaccine inducing the synthesis of anti-cardiolipin antibodies (aCL), anti-β2GPI, lupus anti-coagulant (LA) or other immunoserological phenomena in a healthy young population.
Patients and methods
The study population consisted of 85 healthy medical students (63 female, 22 male; mean age 20·8 years) who were vaccinated with three doses of recombinant DNA hepatitis B vaccine (Engerix-B®, GlaxoSmithKline Biologicals, Rixensart, Belgium). The second and third doses were given 1 and 6 months after the first dose. Each 1-ml adult dose contains 20 µg of purified hepatitis B surface antigen, a major polypeptide of 226 amino acids designated HBs in a non-glycosylated (p24) form adsorbed on the adjuvant aluminium hydroxide.
All participants were surveyed and questioned at the time of each vaccination, especially about intercurrent infections, and they were screened for the presence of IgG and IgM aCL, IgG and IgM anti-β2GPI, LA, anti-nuclear antibodies (ANA) and antibodies against extractable nuclear antigens (anti-ENA). aCL and anti-β2GPI antibodies were determined by our in-house enzyme-linked immunosorbent assays (ELISAs) as described previously [4–6]. The cut-off values were based on the control group comprised 434 healthy blood donors who did not receive the vaccine [5]. At the first and the last vaccinations the participants were also screened for specific antibodies against HBs antigen (Enzygnost Anti-HBs II, Dade Behring, Marburg, Germany).
The LA was detected by coagulation assays, following the guidelines of the International Society on Thrombosis and Haemostasis, using activated partial thromboplastin time test (aPTT), a modified dilute Russell viper venom time test (dRVVT) [6] and a confirmatory test in which the dRVVT-confirm reagent (LA-Confirm, Gradipore, Sydney, Australia) contained excess phospholipids to neutralize the LA effect.
ANA were determined by a standard indirect immunofluorescence technique on HEp-2 cells (Immuno Concepts, Sacramento, CA, USA). ANA of 1 : 80 or higher was considered positive. Anti-ENA antibodies were detected by a standard counter-immunoelectrophoresis using rabbit thymus and human spleen extracts as the antigen substrates [7].
The study was approved by the Ethics’ Committee of the Slovenian Ministry of Health. Informed consent for drawing extra blood at the time of each vaccination was obtained from all participants.
Statistical tests were performed using paired data analyses (Wilcoxon's matched-pairs signed-ranks test corrected for ties, Spearman's rank correlation corrected for ties), χ2 test and t-test assuming equal or unequal variances were used when appropriate. Differences were considered statistically significant when P < 0·05.
Results
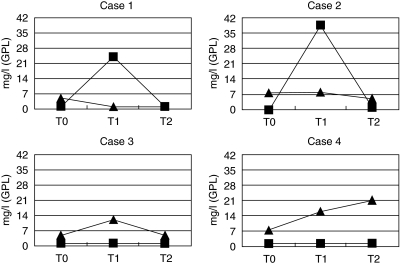
One month after vaccination with the first dose of hepatitis B vaccine a statistically significant number of subjects showed no changes in IgG/IgM aCL, IgG/IgM anti-β2GPI or lupus anti-coagulant activity. Among subjects in whom changes of IgG anti-β2GPI were observed, a significantly higher number of increased (8/85) than decreased (2/85) values were found (P < 0·01), while for IgG aCL no statistically significant differences in the number of increased (8/85) or decreased (11/85) values were detected. The overall increase of the arithmetical mean values of IgG aCL and IgG anti-β2GPI levels were observed 1 month after vaccination with the first dose of hepatitis B vaccine, with a drop in the mean values 5 months later: 5·09, 5·36, 4·7 GPL for aCL and 3·96, 4·14, 3·81 arbitrary units (corresponding to mg/l of HCAL and EY2C9 monoclonal antibodies) for anti-β2GPI. Analyses of paired data did not show statistical significance of differences. In two participants aCL transitorily reached medium positivity after the first dose of hepatitis B vaccine with a drop 5 months later, without history of any intercurrent infection. A similar transient increase has been also observed for anti-β2GPI in one participant. Another student was initially low positive for IgG anti-β2GPI and the levels increased progressively during 6 months’ follow-up after vaccination (Fig. I). There were no sex-dependent differences in tested antibodies observed.
Fig. 1.
Subjects with evident anti-cardiolipin antibodies (aCL) (▪) and/or anti-β2GPI (▴) fluctuation after vaccination (cut-off for aCL = 7 GPL, cut-off for anti-β2GPI = 7·2 mg/l). T0 = time of vaccination, T1 = 1 month after vaccination, T2 = 6 months after vaccination.
Two participants became positive for ANA during 6 months’ follow-up, while in initially positive participants (19/85) ANA did not show any tendency to increase. Only one participant exhibited positive anti-ENA reacting with an unknown antigen from human spleen extract.
Seventy-seven of 85 participants showed an adequate immune response after HepB vaccination with production of protective level of anti-HBs antibodies. Eight participants (9%) showed an inadequate immune response after HepB vaccination (< 10 IU/l) and two participants already had protective levels of anti-HBs before the first vaccination. No correlations were found in the analysis of subsets of aPL positive and anti-HBs positive individuals,
Discussion
Immunization with a recombinant hepatitis B vaccine has been found to be extremely efficient and is integrated into routine immunization schedules worldwide. Together with the universal use of the vaccine, serious adverse effects have been reported, including several autoimmune phenomena [8–10].
Clinically, APS was reported as associated with diverse microbial agents [11]. We followed a group of volunteers in a prospective longitudinal study after hepatitis B vaccination. We were able to demonstrate an induction of aCL and in one participant an induction of anti-β2GPI. The first finding could be in agreement with previous knowledge of infection-induced aCL, resulting in an overall consensus that aCL should be tested twice with an interval of at least 8 weeks. There is growing clinical and experimental evidence that aPL can be induced by various infectious agents [12]. The prevalence and clinical significance of aPL have been studied intensively in patients with chronic hepatitis C virus (HCV) infection. Low levels of aCL were frequently found in patients with HCV infection, but were only rarely associated with the development of thrombotic events [13]. Data on aPL in patients with hepatitis B virus (HBV) infection are very rare and only two studies reported that the prevalence of aPL in HBV patients was lower than in patients with HCV infection [14,15].
Several mechanisms may be involved in the induction of aPL by viral infections and viral vaccination: bystander mechanisms, the exposure of normally hidden epitopes due to tissue destruction, polyclonal activation of B cells, including some autoreactive and molecular mimicry. HbsAg-stimulated peripheral blood mononuclear cells from hepatitis B vaccines synthesized high levels of interferon-γ but little or no interleukin-4 and -10 [16]. It has been suggested that there are ‘windows of opportunity’ during which changes in endogenous cytokine activity can demonstrate profound effects on (autoimmune) disease and its progression [17]. A transient increase in autoantibody production after immunization has been observed in dogs [18], mice [19], baboons and humans [20]. These autoantibodies were considered benign. However, the fact that autoantibodies occasionally remain elevated (as in one case in our study) suggests that in some patients vaccine-induced autoantibodies could become pathological [10]. For the induction of an overt autoimmune condition, the genetic background (most probably HLA) combined with the vaccines to load such an event is probably necessary. Another involved mechanism could be molecular mimicry, where an antigen of the recombinant vaccine forms immunogenic complexes against which aCL and anti-β2GPI antibodies are produced. A similar mechanism of anti-β2GPI induction has already been established after immunization with tetanus toxoid in experimental animal models [1]. Epitope mimicry as a possible mechanism for induction of aPL seems particularly relevant for one of our participants, who demonstrated a progressive increase of IgG anti-β2GPI antibodies after repeated vaccinations. It is also possible that the adjuvant is responsible for the induction of autoantibodies and not the infectious component of the vaccine [3]. None of our study participants demonstrated an induction of the synthesis of any other autoantibodies tested, therefore such an explanation seems less likely concerning our results. Finally, concomitant exposure to some viral infection, together with the vaccination, might have had an adjuvant effect on the induction of aCL and anti-β2GPI, but no correlations were found between infections during the study and the appearance or the levels of aPL. In spite of the fact that our results generally do not support a clear-cut influence of hepatitis B vaccine on aPL induction, we found that HBV can rarely induce production of aPL. In genetically susceptible individuals or together with some other triggers such a combination might confer the risk of developing a continuous autoimmune response in an individual.
References
- 1.Blank M, Krause I, Fridkin M, et al. Bacterial induction of autoantibodies to β2-glycoprotein I accounts for the infectious ethiology of the antiphospholipid syndrome. J Clin Invest. 2002;109:797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gharavi AE, Pierangeli SS, Espinola RG, Liu X, Colden-Stanfield M, Harris EN. Antiphospholipid antibodies induced in mice by immunization with cytomegalovirus-derived peptide cause thrombosis and activation of endothelial cells in vivo. Arthritis Rheum. 2002;46:545–52. doi: 10.1002/art.10130. [DOI] [PubMed] [Google Scholar]
- 3.Shoenfeld Y, Aron-Maor A. Vaccination and autoimmunity – ’vaccinosis’: a danger liaison? J Autoimmun. 2000;14:1–10. doi: 10.1006/jaut.1999.0346. [DOI] [PubMed] [Google Scholar]
- 4.Stegnar M, Božič B, Peternel P, Kveder T, Vene N, Rozman B. Prevalence of antiphospholipid antibodies in deep vein thrombosis and their relationship to blood coagulation and fibrinolysis. Thromb Res. 1991;63:433–43. doi: 10.1016/0049-3848(91)90230-t. [DOI] [PubMed] [Google Scholar]
- 5.Čučnik S, Ambrožič A, Božič B, Skitek M, Kveder T. Anti-beta2-glycoprotein I ELISA: methodology, determination of cut-off values in 434 healthy Caucasians and evaluation of monoclonal antibodies as possible international standards. Clin Chem Lab Med. 2000;38:777–83. doi: 10.1515/CCLM.2000.111. [DOI] [PubMed] [Google Scholar]
- 6.Avčin T, Ambrožič A, Božič B, Accetto M, Kveder T, Rozman B. Estimation of anticardiolipin antibodies, anti-beta2 glycoprotein I antibodies and lupus anticoagulant in a prospective longitudinal study of children with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2002;20:101–8. [PubMed] [Google Scholar]
- 7.Bunn C, Kveder T. Counterimmunoelectrophoresis and immunodiffusion for the detection of antibodies to soluble cellular antigens. In: Van Venrooij WJ, Maini RN, editors. Manual of biological markers of disease. A3. Dordrecht: Kluwer Academic Publishers; 1993. pp. 1–12. [Google Scholar]
- 8.Maillefert JF, Sibilia J, Toussirot E, et al. Rheumatic disorders developed after hepatitis B vaccination. Rheumatology. 1999;38:978–83. doi: 10.1093/rheumatology/38.10.978. [DOI] [PubMed] [Google Scholar]
- 9.Geier MR, Geier DA, Zahalsky AC. A review of hepatitis B vaccination. Exp Opin Drug Saf. 2003;2:113–22. doi: 10.1517/14740338.2.2.113. [DOI] [PubMed] [Google Scholar]
- 10.Borchers AT, Keen CL, Shoenfeld Y, Silva J, Gershwin ME. Vaccines, viruses, and voodoo. J Invest Allergol Clin Immunol. 2002;12:155–68. [PubMed] [Google Scholar]
- 11.Cervera R, Asherson RA, Acevedo ML, et al. Antiphospholipid syndrome associated with infections: clinical and microbiological characteristics of 100 patients. Ann Rheum Dis. 2004;63:1312–7. doi: 10.1136/ard.2003.014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blank M, Shoenfeld Y. Beta-2-glycoprotein-I, infections, antiphospholipid syndrome and therapeutic considerations. Clin Immunol. 2004;112:190–9. doi: 10.1016/j.clim.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Ordi-Ros J, Villarreal J, Monegal F, Sauleda S, Esteban I, Vilardell M. Anticardiolipin antibodies in patients with chronic hepatitis C virus infection: characterization in relation to antiphospholipid syndrome. Clin Diagn Lab Immunol. 2000;7:241–4. doi: 10.1128/cdli.7.2.241-244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sthoeger ZM, Fogel M, Smirov A, et al. Anticardiolipin autoantibodies in serum samples and cryoglobulins of patients with chronic hepatitis C infection. Ann Rheum Dis. 2000;59:483–6. doi: 10.1136/ard.59.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada M, Fujisawa Y, Sakisaka S, et al. High prevalence of anticardiolipin antibodies in hepatitis C virus infection: lack of effects on thrombocytopenia and thrombotic complications. J Gastroenterol. 2000;35:272–7. doi: 10.1007/s005350050345. [DOI] [PubMed] [Google Scholar]
- 16.Rahman F, Dahmen A, Herzog-Hauff S, Bocher WO, Galle PR, Lohr HF. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31:521–7. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 17.Borchers AT, Kuhl SJ, Keen CL, Meroni PL, Gershwin ME. Cytokines, chemokines, and their cognate receptors in autoimmune diseases. Sem Clin Immunol. 1999;18:17–39. [Google Scholar]
- 18.Hogenesch H, Azcona-Olivera J, Scott-Monterieff C, Snyder PW, Glickman LT. Vaccine-induced autoimmunity in the dog. Adv Vet Med. 1999;41:733–47. doi: 10.1016/s0065-3519(99)80056-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Morgan-Capmer P, Latif N, et al. Coxsackie virus B3-induced myocarditis. Characterisation of stable attenuated variants that protect against infection with cardiovirulent wild-type strain. Am J Pathol. 1997;150:2197–207. [PMC free article] [PubMed] [Google Scholar]
- 20.Rose NR. Immunologic hazards associated with vaccination of humans. J Autoimmun. 2000;14:11–3. doi: 10.1006/jaut.1999.0347. [DOI] [PubMed] [Google Scholar]