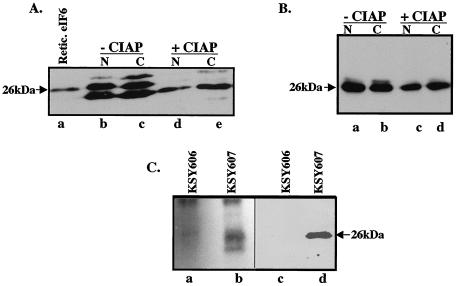
FIG. 1.
eIF6 is phosphorylated in HeLa and yeast cells. (A) Cell extracts of HeLa cells were fractionated into nuclear (N) and cytoplasmic (C) fractions. Where indicated, aliquots of each fraction were preincubated with calf intestinal alkaline phosphatase (CIAP). These fractions (lanes d and e) and untreated controls (lanes b and c) along with purified rabbit reticulocyte eIF6 (Retic. eIF6; lane a) were subjected to Western blot analysis by using anti-mammalian eIF6 antibodies as probes. (B) Nuclear and cytoplasmic fractions derived from extracts of KSY607 yeast cells, expressing HA-tagged Tif6p, were preincubated either with calf intestinal alkaline phosphatase (lanes c and d) or with buffer (lanes a and b) and then subjected to Western blot analysis by using anti-HA antibodies as probes. (C) Cell extracts of 32P-labeled yeast cells expressing either HA-Tif6p (strain KSY607) or untagged Tif6p (strain KSY606) were immunoprecipitated with anti-HA antibodies. The washed immunocomplexes were subjected to SDS-PAGE followed by electrophoretic transfer onto a polyvinylidene difluoride membrane. The blot was first subjected to autoradiography (lanes a and b) and then analyzed by immunoblotting by using anti-HA antibodies (lanes c and d). The faster-migrating band in lane b may represent a degradation product of Tif6p.