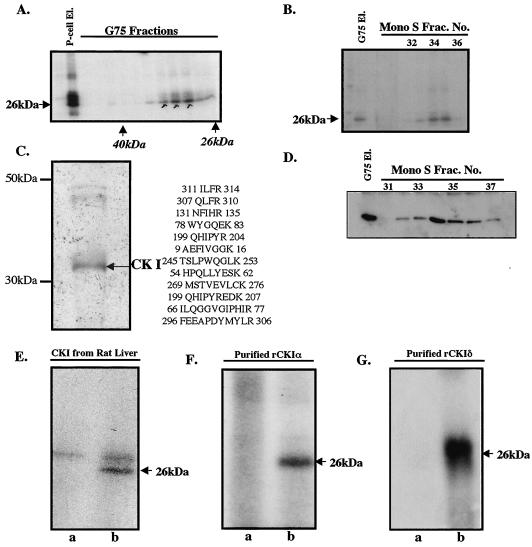
FIG. 2.
Purification and characterization of eIF6 kinase from rabbit reticulocyte lysates. (A) A partially purified eIF6 kinase preparation (dialyzed ammonium sulfate fraction derived from the phosphocellulose eluate [P-cell El.]) was subjected to Sephadex G-75 gel filtration as described in Materials and Methods. Aliquots of the protein fractions eluted from the column were assayed for eIF6 kinase activity. (B) The pooled Sephadex G-75 eluate [G75 El.] of eIF6 kinase was subjected to FPLC on a Mono S column. Aliquots of each fraction as well as the pooled G-75 fraction that was loaded onto the Mono S column were assayed for eIF6 kinase activity. Frac. No., fraction number. (C) The pooled Mono S fraction was analyzed by SDS-PAGE followed by silver staining. The major polypeptide band was digested with trypsin and analyzed by mass spectrometry. The sequenced peptides that correspond to CK Iα are indicated. (D) Fractions eluting from the FPLC Mono S column that contained eIF6 kinase activity were analyzed by Western blotting by using anti-mammalian CK Iα antibodies. (E, F, and G) Recombinant human eIF6 (3 pmol) was phosphorylated by using [γ-32P]ATP and either purified rat liver CK I (E), a purified recombinant mammalian CK Iα isoform (purified rCKIα) (F), or purified recombinant mammalian CK Iδ (purified rCKIδ) (G). In each case, the reaction mixture in lane a did not contain eIF6 while the reaction mixture in lane b was the complete system containing eIF6. In panel E, the slower-migrating bands present in both lanes a and b represent autophosphorylated CK I.