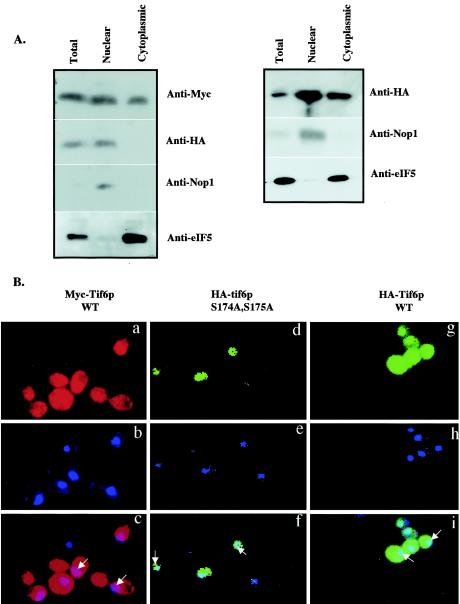
FIG. 6.
Subcellular distribution of wild-type Tif6p and the serine phosphorylation mutant of Tif6p. (A, left panel) Lysates of haploid yeast strain UBY608 carrying an inactive chromosomal TIF6 gene and harboring both the TRP1 plasmid pRS314-mycTIF6 (which expresses Myc-tagged Tif6p from its natural promoter) and a recombinant LEU2 plasmid pRS315-TIF6 (S174A, S175A), which expresses mutant HA-Tif6p protein (S174A, S175A) from its natural promoter, were fractionated into postnuclear supernatant (cytosolic fraction) and nuclear pellet as described by Aris and Blobel (1). (Right panel) Similar analysis was carried out with lysates of yeast strain UBY610 expressing both the Myc-tagged Tif6p and theHA-tagged wild-type Tif6p from its natural promoter. Approximately 30 μg of protein from each fraction was subjected to Western blot analysis by using anti-Myc, anti-HA, anti-Nop1, and anti-eIF5 antibodies as probes. (B) Localization of wild-type Myc-Tif6p and wild-type and mutant HA-Tif6p fusion proteins. Indirect immunofluorescence staining was performed with UBY608 cells expressing both the wild-type (WT) Myc-Tif6p (panels a to c) and the mutant HA-Tif6p fusion proteins (panels d to f). Similar indirect immunofluorescence staining was also carried out with UBY610 cells expressing both wild-type Myc-Tif6p and wild-type HA-Tif6p from two different CEN plasmids. Chromatin DNA of yeast cells was stained with DAPI. HA-Tif6p (d and g) was detected by immunofluorescence staining with monoclonal mouse anti-HA antibody and anti-mouse antibodies conjugated with Alexa Fluor 488 (Molecular Probes, Eugene, Oreg.). For Myc-Tif6p (a), the primary antibody used was anti-Myc antibodies and the secondary antibody used was anti-rabbit antibodies conjugated with Alexa Fluor 568 (Molecular Probes). In the third panels (c, f, and i), the two top panels (a and b, d and e, and g and h, respectively) were merged. Arrows indicate the positions of the nuclei.